-
Applications
-
Life Science
- rapidFLIMHiRes (Fluorescence Lifetime Imaging) New
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Foerster Resonance Energy Transfer (FRET)
- Pulsed Interleaved Excitation (PIE)
- scanning Fluorescence Correlation Spectroscopy (sFCS) New
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Dual-focus Fluorescence Correlation Spectroscopy (2fFCS)
- Stimulated Emission Depletion Microscopy (STED)
- Single Molecule Detection
- Time-resolved Fluorescence
- Fluorescence Anisotropy (Polarization)
- Pattern Matching Analysis
- Two-Photon Excitation (TPE)
- Diffuse Optical Tomography and Imaging
- Singlet Oxygen
- Laser Cutting/Ablation
- Materials Science
- Metrology
- Quantum Optics
-
Life Science
Life Science
Fluorescence Anisotropy (Polarization)
Studying molecular orientation and mobility as well as the factors influencing them
Measurement of steady-state and particularly time-resolved fluorescence anisotropy offers fascinating possibilities to study molecular orientation and mobility as well as processes that affect them. Except of special cases, anisotropy does not depend on the concentration of fluorophores, thus on the detected signal intensity. Owing to this similarity with the behavior of fluorescence lifetime, we can regard anisotropy as a yet another dimension of fluorescence information.
Molecules with their absorption transition moment (ATM) aligned with the polarization plane of excitation are preferentially excited. This is called photo-selection, because at the same time molecules with ATM oriented perpendicular to the excitation polarization plane are not excited, they remain in their ground state.
Subsequently, the polarization plane of an emitted fluorescence photon is defined by the orientation of the emission transition moment of the molecule, thus by the orientation of the molecule itself at the moment of emission. The degree of fluorescence polarization expressed as a dimensionless quantity, anisotropy, is usually the highest at the moment of excitation and then normally decreases in time. Common reason for this is the random molecular motion, e.g. Brownian rotation or conformational flexibility that tends to randomize the initially well aligned, photoselected fluorophore population. The so called anisotropy decay can be observed sometimes even in a solid sample (e.g. rigid, frozen or very viscous environment) owing to intramolecular processes or energy transfer between molecules. Time-resolved anisotropy measurement is more informative than its steady-state counterpart, the latter reporting time averaged values only, without direct insight into the dynamics of the process.
more...
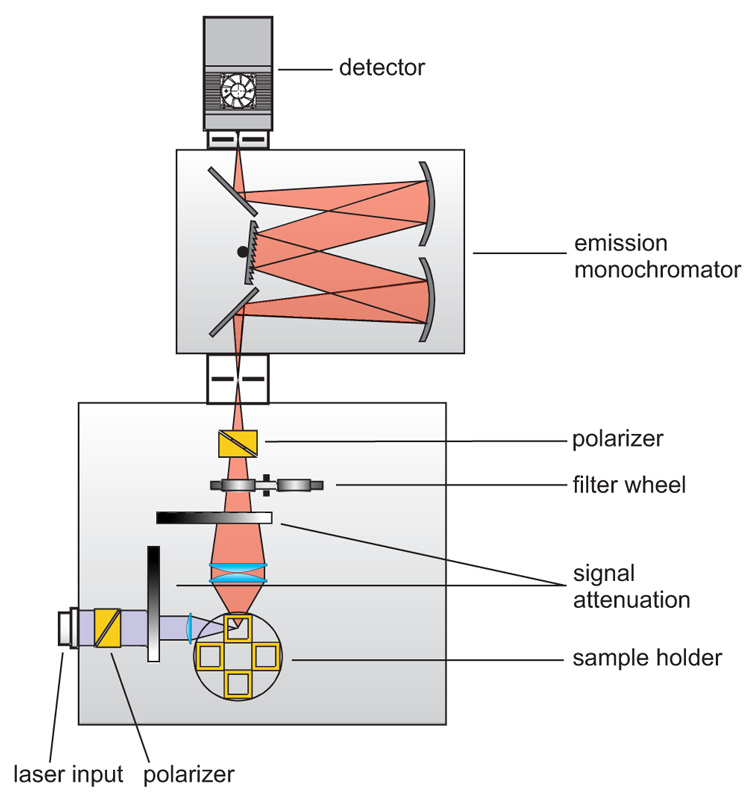
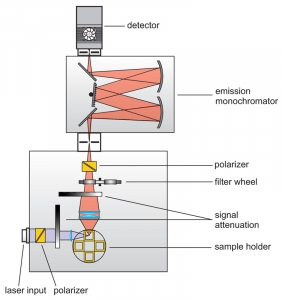 Anisotropy measurements are typically performed with standard fluorescence spectrometers using plane-polarized excitation light. Lasers usually emit polarized light; the output of LEDs or steady-state lamps is made polarized using high quality polarizers. The fluorescence emission is then detected through a second polarizer (sometimes called analyzer). In standard fluorescence spectrometers the excitation is usually polarized vertically in respect to the plane of the optics (L-geometry). Anisotropy (-decay) is then calculated from consecutively measured vertical and horizontal polarized intensities (decays). In microscopes it is more common to use two detectors and a polarizing beam splitter inserted between them.
Anisotropy measurements are typically performed with standard fluorescence spectrometers using plane-polarized excitation light. Lasers usually emit polarized light; the output of LEDs or steady-state lamps is made polarized using high quality polarizers. The fluorescence emission is then detected through a second polarizer (sometimes called analyzer). In standard fluorescence spectrometers the excitation is usually polarized vertically in respect to the plane of the optics (L-geometry). Anisotropy (-decay) is then calculated from consecutively measured vertical and horizontal polarized intensities (decays). In microscopes it is more common to use two detectors and a polarizing beam splitter inserted between them.
Consequently the essential components of a spectrometer set-up capable of anisotropy measurements are:
- pulsed (and/or CW) excitation source with plane-polarized excitation light
- single photon sensitive detector
- emission polarizer (analyzer)
- monochromator or optical filters
- TCSPC or MCS unit to measure the time between excitation and fluorescence emission
Related technical and application notes:
Steady-sate and time-resolved gluorescence anisotropy can be investigated with the following PicoQuant systems
Single Photon Counting Confocal Microscope
Luminosa combines state-of-the-art hardware with cutting edge software to deliver highest quality data while simplifying daily operation. The software includes context-based workflows for FLIM, FRET, and FCS, which allow the users to focus on their samples. Tight integration of hardware and software enable automated routines, e.g., for auto-alignment or laser power calibration, which increase ease of use as well as reproducibility of experiments. Still, an open mode of operation is available for full access to every optomechanical component via software.
High performance fluorescence lifetime spectrometer
The FluoTime 300 "EasyTau" is a fully automated, high performance fluorescence lifetime spectrometer with steady-state and phosphorescence option. The system features an ultimate sensitivity with a record breaking 24.000:1 Water Raman SNR. The FluoTime 300 contains the complete optics and electronics for recording fluorescence decays by means of Time-Correlated Single Photon Counting (TCSPC) or Multichannel Scaling (MCS). The system is designed to be used with picosecond pulsed diode lasers, LEDs or Xenon lamps. Multiple detector options enable a large range of system configurations. The FluoTime 300 can be used to study fluorescence and phosphorescence decays between a few picoseconds and several seconds
Compact and modular fluorescence lifetime spectrometer
The FluoTime 250 integrates all essential optics and electronics for time-resolved luminescence spectroscopy in a compact, fully automated device. This spectrometer is designed to assist the user in carrying out routine as well as complex measurements easily and with high reliability thanks to fully automated hardware components and a versatile system software featuring wizards providing step-by-step guidance.
Modular, time-resolved confocal fluorescence microscopy platform
The MicroTime 200 time-resolved fluorescence microscope system is a powerful instrument capable of Fluorecence Corelation Spectroscopy and its daughter techniques as well as Fluorescence Lifetime Imaging (FLIM) with single molecule detection sensitivity. It contains the complete optics and electronics for recording virtually all aspects of the fluorescence dynamics of microscopic samples or femtoliter volumes. The instrument gains its exceptional sensitivity and flexibility in combination with unprecedented ease-of-use from a unique fusion of miniaturized and highly sophisticated state-of-the-art technologies. Although, these technologies enable to run an instrument of comparable complexity and power without having to spend more time on instrument maintenance than on original scientific content, the MicroTime200 remains an open platform that allows the advanced scientist to easily built upon the open character of the instrument in order to realize highly customized applications
LSM Upgrade Kits from PicoQuant add FLIM capabilities to laser scanning microscopes from many major manufactures
Confocal Laser Scanning Microscopes (LSMs) are widely used tools in cell and molecular biology, biochemistry and other related sciences. PicoQuant's LSM Upgrade Kits greatly enhance the capabilities of these microscopes by extension to time-resolved techniques, and thereby providing not only Fluorescence Lifetime Imaging (FLIM) but also Fluorescence Correlation Spectroscopy (FCS) and a wealth of other time resolved techniques. The LSM Upgrade Kit combines PicoQuant products to a ready-to-use kit that fits your specific application on a state of the art Laser Scanning Microscope of your choice from Leica, Nikon, Olympus or Zeiss.
 The following core components are needed to build a system capable of time-resolved fluorescence anisotropy measurements, which are (partly) available from PicoQuant:
The following core components are needed to build a system capable of time-resolved fluorescence anisotropy measurements, which are (partly) available from PicoQuant:
- Laser drivers
- Laser or LED heads
- Photon Counting Detector
- TCSPC and MCS Electronics
- Analysis software
- Monochromator or optical filters
- Polarizers
- Focussing and collection optics
Applications for time-resolved anisotropy measurements include:
- Investigations of biomembrane fluidity and rigidity
- Local microviscosity in liquids and polymers
- Protein-protein interactions (crowding, oligomerization)
- Receptor-ligand binding
- Myosin reorientation
- Monitoring FRET between identical molecules (homo-FRET)
- Alignment of molecules in various matrices
Investigating the dynamic anisotropy of Coumarin 6
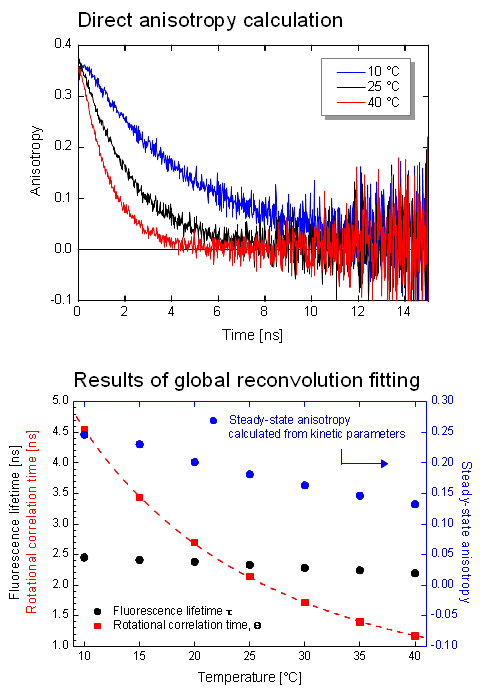
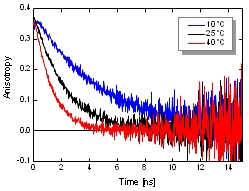
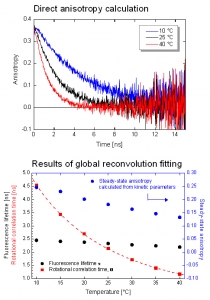 The dynamic anisotropy of Coumarin 6 was studied using the FluoTime 300. The system automatically set the sample temperature, and at each temperature step, four measurements were automatically performed: IRF and VV (parallel), VH (perpendicular), and VM (magic angle) polarized decay measurements. A quick analysis of VV and VH decays clearly shows temperature dependent behavior of the emission anisotropy. For detailed quantitative results, global reconvolution anisotropy analysis of the data set was performed. A model of a single, spherical, rotating particle with a single exponential fluorescence lifetime proved to describe the system very well, as expected for a small, highly polar particle in polar solvent. The result reveals the slightly temperature dependent single exponential fluorescence lifetime of Coumarin 6. The temperature dependence of the viscosity could be fitted to the experimentally obtained change of the rotation correlation times. Even a precise calculation of the steady-state anisotropy values was possible which were found to be in perfect agreement with the anisotropy values estimated by the Perrin equation.
The dynamic anisotropy of Coumarin 6 was studied using the FluoTime 300. The system automatically set the sample temperature, and at each temperature step, four measurements were automatically performed: IRF and VV (parallel), VH (perpendicular), and VM (magic angle) polarized decay measurements. A quick analysis of VV and VH decays clearly shows temperature dependent behavior of the emission anisotropy. For detailed quantitative results, global reconvolution anisotropy analysis of the data set was performed. A model of a single, spherical, rotating particle with a single exponential fluorescence lifetime proved to describe the system very well, as expected for a small, highly polar particle in polar solvent. The result reveals the slightly temperature dependent single exponential fluorescence lifetime of Coumarin 6. The temperature dependence of the viscosity could be fitted to the experimentally obtained change of the rotation correlation times. Even a precise calculation of the steady-state anisotropy values was possible which were found to be in perfect agreement with the anisotropy values estimated by the Perrin equation.
Set-up:
- FluoTime 300
- Excitation: 440 nm
- Emission detected at 510 nm with 3 nm bandpass
- Analysis: FluoFit (replaced by EasyTau 2)
Recording the fluorescence anisotropy of Apomyoglobin in buffer
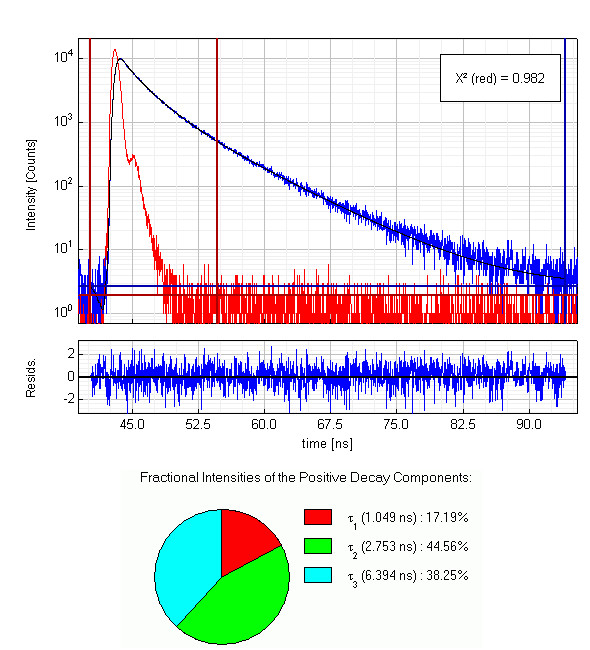
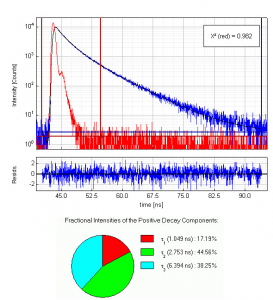 Full length apomyoglobin has 154 amino acids including two tryptophans and three tyrosines. A 5 µM solution of this protein in acetate buffer was excited at 280 nm. Glan-Taylor polarisers where used in order to select the vertically polarized component of the excitation light and the magic-angle polarized component of the fluorescence. The collected emission passed a monochromator set to 350 nm with 30 nm bandpass. The measured instrument response function (IRF) has a FWHM of 700 ps and 1.2 million counts were collected within 4 minutes measurement time. The analysis of the measurement results showed that in this case the decay is best described by a triple exponential decay funciton. The picture shows the IRF (red), the measured decay (blue) and the result of the fit (black) along with the three recovered lifetimes and their fractional intensities.
Full length apomyoglobin has 154 amino acids including two tryptophans and three tyrosines. A 5 µM solution of this protein in acetate buffer was excited at 280 nm. Glan-Taylor polarisers where used in order to select the vertically polarized component of the excitation light and the magic-angle polarized component of the fluorescence. The collected emission passed a monochromator set to 350 nm with 30 nm bandpass. The measured instrument response function (IRF) has a FWHM of 700 ps and 1.2 million counts were collected within 4 minutes measurement time. The analysis of the measurement results showed that in this case the decay is best described by a triple exponential decay funciton. The picture shows the IRF (red), the measured decay (blue) and the result of the fit (black) along with the three recovered lifetimes and their fractional intensities.
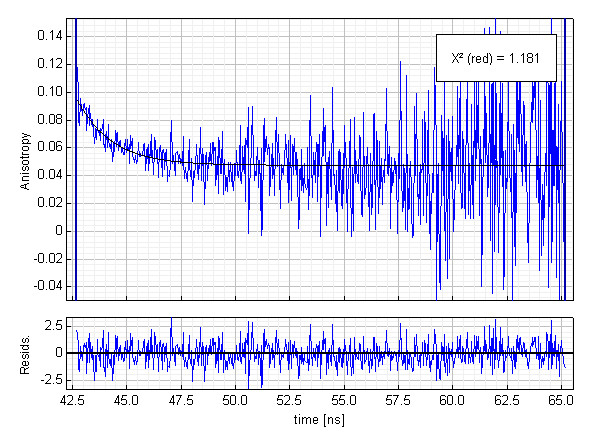
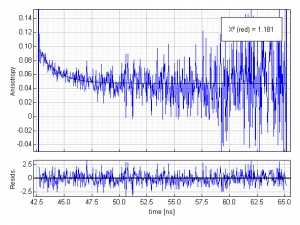 Further information about the protein dynamics can be obtained by a quick anisotropy decay analysis. Two more histograms were collected, the so called parallel and perpendicular polarized decays. The simplest anisotropy analysis involves a direct calculation of the anisotropy decay curve R(t) from those polarized decays. The necessary G-factor was determined in an independent decay measurement with horizontally polarized excitation.The picture shows the time evolution of the anisotropy (blue) during the first 20 ns and a fitted single exponential decay curve (black). The time resolved anisotropy reveals an average rotational correlation time of 1.6 ns and a residual anisotropy of 0.05. This residual anisotropy is a clear indication of a slower process, which cannot be resolved using the fluorescence of tyrosine and tryptophane as an indicator. In fact, the expected rotational correlation time of apomyoglobin is in the order of 7-8 ns and this process is most likely the reason for the residual anisotropy.
Further information about the protein dynamics can be obtained by a quick anisotropy decay analysis. Two more histograms were collected, the so called parallel and perpendicular polarized decays. The simplest anisotropy analysis involves a direct calculation of the anisotropy decay curve R(t) from those polarized decays. The necessary G-factor was determined in an independent decay measurement with horizontally polarized excitation.The picture shows the time evolution of the anisotropy (blue) during the first 20 ns and a fitted single exponential decay curve (black). The time resolved anisotropy reveals an average rotational correlation time of 1.6 ns and a residual anisotropy of 0.05. This residual anisotropy is a clear indication of a slower process, which cannot be resolved using the fluorescence of tyrosine and tryptophane as an indicator. In fact, the expected rotational correlation time of apomyoglobin is in the order of 7-8 ns and this process is most likely the reason for the residual anisotropy.
Set-up:
- FluoTime 200
- Excitation: PLS 280, 2.5 MHz repetition rate
- Analysis: FluoFit (replaced by EasyTau 2)
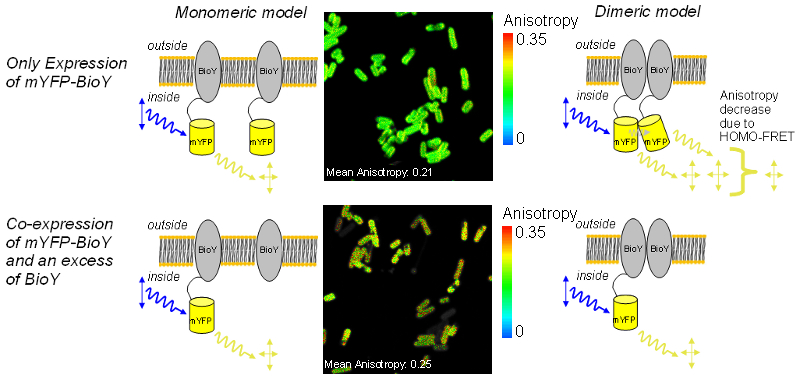
Using anisotropy imaging to study the interaction of the ECF-transporter subunit BioY
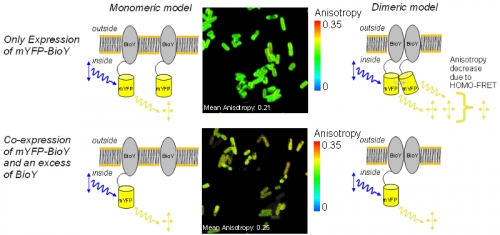 ECF-transporters present in most bacteria are responsible for the uptake of vitamins, micronutrients and intermediate products. The importers consist of three components: a substrate specific membrane protein (S-unit), an integral membrane spanning part (T-unit) and a nucleotid binding protein with ATP-binding site (ABC). Whilst T-unit and ATPase are conserved, the S-subunit varies giving the whole transporter its name “Energy Coupling Factor”, ECF.
ECF-transporters present in most bacteria are responsible for the uptake of vitamins, micronutrients and intermediate products. The importers consist of three components: a substrate specific membrane protein (S-unit), an integral membrane spanning part (T-unit) and a nucleotid binding protein with ATP-binding site (ABC). Whilst T-unit and ATPase are conserved, the S-subunit varies giving the whole transporter its name “Energy Coupling Factor”, ECF.
The S-unit responsible for biotin-uptake (BioY) is also active without T and ATPase parts. Therefore, interactions between BioY units were expected. HOMO-FRET between mYFP-BioY was used in E. coli mutant to prove dimerization. A decreased mean anisotropy of 0.21 was indicative of HOMO-FRET in bacteria containing only labeled BioY (see upper image).
As a control, in another variant, mYFP-BioY was expressed along with an excess of unlabeled BioY. This caused a reduction of HOMO-FRET and an increase in anisotropy towards 0.25 (lower image). With these experiments a dimerization of BioY could be demonstrated.
The anisotropy mean values of 0.25 and 0.21 were measured on a Fluorescence Lifetime Spectrometer FluoTime 200 in suspension. The G-Factor of the microscope was calibrated in a way that the mean anisotropy of the high anisotropy cell line matched the spectrometer value. When doing this, the mYFP-BioY expressing bacteria showed an anisotropy value matching the spectrometer value of 0.21.
Set-up:
- Olympus FluoView FV1000 with LSM Upgrade Kit and extension for polarization measurements
- Excitation: 485 nm, 10 MHz repetition rate
- Emission: 520 – 560 nm, dual channel set-up for parallel and perpendicular polarization detection
- Analysis: SymPhoTime 64
Courtesy of Joanna Ziomkowska and Andreas Herrmann, Molecular Biophysics group, Humboldt University, Berlin, Germany
Reference: Ziomkowska et al., BIOspectrum, 5, 498 (2012)
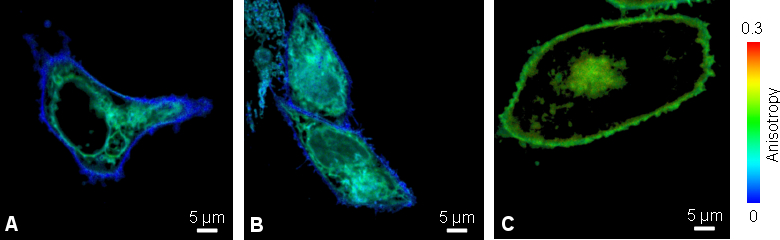
Fluorescence anisotropy imaging enables study of oligomerization of viral membrane proteins
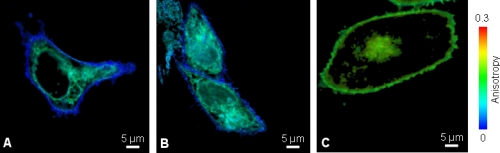 A specific viral membrane protein (v.m.p.) which is part of HIV protein, is responsible for membrane fusion of the HI virus with the T-cell membrane. After infection and synthesis it is known to be transported to the plasma membrane via intracellular transport vesicles, Golgi apparatus and ER.
A specific viral membrane protein (v.m.p.) which is part of HIV protein, is responsible for membrane fusion of the HI virus with the T-cell membrane. After infection and synthesis it is known to be transported to the plasma membrane via intracellular transport vesicles, Golgi apparatus and ER.
Living Chinese Hamster Ovary (CHO) cells transfected with v.m.p-mYFP showed a lower anisotropy value at outer cell membrane compared to the intracellular protein localization (Fig. A). To clarify if this decrease was caused by either clustering or different mobility of the viral membrane protein at the cell surface, the cells were stained with an anti-GFP antibody to increase the molecular weight of YFP (Fig B). This would slow down the molecular rotation of YFP sticking out of the membrane into the extracellular space and resulting in an anisotropy change. Since the value was unaltered upon antibody binding, the lower anisotropy of v.m.p.-mYFP at the outer cell membrane was caused by HOMO-FRET due to clustering of the viral membrane protein.
As a negative control, the anisotropy was imaged in CHO cells expressing GPI-mYFP (Fig. C). Glycosyl-phosphatidyl-inositol anchors are mainly localized at the cellular plasma membrane, do not interact or cluster and thus show a higher anisotropy compared to the viral membrane protein.
Set-up:
- Olympus FluoView FV1000 with LSM Upgrade Kit and extension for polarization measurements
- Excitation: 470 nm, 10 MHz repetition rate
- Emission: 520 – 560 nm, dual channel set-up for parallel and perpendicular polarization detection
- Analysis: SymPhoTime 64
Data courtesy of Roland Schwarzer and Andreas Herrmann, Molecular Biophysics group, Humboldt University, Berlin, Germany
Reference: R. Schwarzer et. al., Cell Microbiol. 2014 May 20. doi: 10.1111/cmi.12314
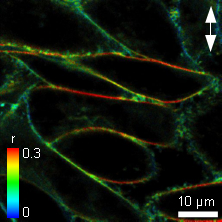
In-cell fluorescence anisotropy measurements of the membrane structure reporter probe C6-NBD-PC
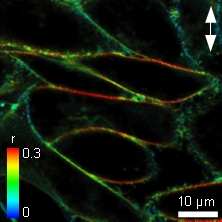 The Polycholin-attached (PC) NBD dye is often used to study membrane structure and dynamics. Adherent Chinese Hamster Ovary (CHO) cells were labeled with C6-NBD-PC staining the cell membrane and their anisotropy was measured. The polarization direction of the laser is marked as a white arrow. In the liquid ordered phase of membranes, NBD has a longer fluorescence lifetime corresponding to less water exposure, and the anisotropy is significantly higher at the horizontal versus the vertical membranes as shown in the Figure. Here the dye molecules have a strict orientation in respect to the membrane and show only restricted rotational movements.
The Polycholin-attached (PC) NBD dye is often used to study membrane structure and dynamics. Adherent Chinese Hamster Ovary (CHO) cells were labeled with C6-NBD-PC staining the cell membrane and their anisotropy was measured. The polarization direction of the laser is marked as a white arrow. In the liquid ordered phase of membranes, NBD has a longer fluorescence lifetime corresponding to less water exposure, and the anisotropy is significantly higher at the horizontal versus the vertical membranes as shown in the Figure. Here the dye molecules have a strict orientation in respect to the membrane and show only restricted rotational movements.
In contrast, in the liquid disordered phase the fluorescence lifetime of the NBD probe would be relatively low. Also the anisotropy difference between the horizontal and vertical membrane would be relatively small, as the fluorescent molecules have more degrees of freedom in orientation and/or for rotation.
As the horizontal cell membranes have a high anisotropy, while vertical membranes have a lower value, this corresponds to the liquid ordered phase.
Set-up:
- Olympus FluoView FV1000 with LSM Upgrade Kit and extension for polarization measurements
- Excitation: 485 nm, 10 MHz repetition rate
- Emission: 520 – 560 nm, dual channel set-up for parallel and perpendicular polarization detection
- Analysis: SymPhoTime 64
How does fluorescence anisotropy work?
Every fluorescent molecule has an electronic transition dipole moment (ETDM) and will be preferably excited when its ETDM is aligned with the excitation’s polarization plane . Subsequently, the polarization of an emitted photon is defined by the orientation of the ETDM (i.e. by the orientation of molecule) at the moment of emission. Measuring this degree of polarization and its temporal change allows studying molecular orientation and mobility as well as the factors influencing them.
What is fluorescence anisotropy?
Fluorescence anisotropy is a dimensionless quantity that expresses the polarization degree of light emitted by fluorophores. It is usually the highest at the moment of excitation and then normally decreases in time. Common reasons for this are random molecular motion, e.g., Brownian rotation or conformational flexibility that will randomize the initially well aligned, photoselected fluorophore population.
When is the use of fluorescence anisotropy helpful?
Measuring steady-state and particularly time-resolved fluorescence anisotropy offers fascinating possibilities to study molecular orientation and mobility as well as processes that affect them.
What is the difference between steady-state and time-resolved fluorescence anisotropy?
Time-resolved anisotropy measurements are often more informative than steady-state experiments. The latter report only time averaged anisotropy values and thus no direct insight into the dynamics of the process.
Latest 10 publications related to Anisotropy
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this application and our products in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.

 Contact us
Contact us Luminosa
Luminosa FluoTime 300
FluoTime 300
 MicroTime 200
MicroTime 200 LSM Upgrade Kit
LSM Upgrade Kit