-
Applications
-
Life Science
- rapidFLIMHiRes (Fluorescence Lifetime Imaging) New
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Foerster Resonance Energy Transfer (FRET)
- Pulsed Interleaved Excitation (PIE)
- scanning Fluorescence Correlation Spectroscopy (sFCS) New
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Dual-focus Fluorescence Correlation Spectroscopy (2fFCS)
- Stimulated Emission Depletion Microscopy (STED)
- Single Molecule Detection
- Time-resolved Fluorescence
- Fluorescence Anisotropy (Polarization)
- Pattern Matching Analysis
- Two-Photon Excitation (TPE)
- Diffuse Optical Tomography and Imaging
- Singlet Oxygen
- Laser Cutting/Ablation
- Materials Science
- Metrology
- Quantum Optics
-
Life Science
Life Science
Fluorescence Lifetime Imaging (FLIM)
An imaging technique that uses fluorescence lifetime of fluorophores to generate additional contrast
Fluorescence Lifetime Imaging (FLIM) produces an image based on the differences in the excited state decay rate from a fluorescent sample. Thus, FLIM is a fluorescence imaging technique where the contrast is based on the lifetime of individual fluorophores rather than their emission spectra. The fluorescence lifetime is defined as the average time that a molecule remains in an excited state prior to returning to the ground state by emitting a photon.
As the fluorescence lifetime does not depend on concentration, absorption by the sample, sample thickness, photo-bleaching and/or excitation intensity it is more robust than intensity based methods. At the same time, the fluorescence lifetime depends on a wealth of environmental parameters such as pH, ion or oxygen concentration, molecular binding or the proximity of energy acceptors making it the technique of choice for functional imaging of many kinds.
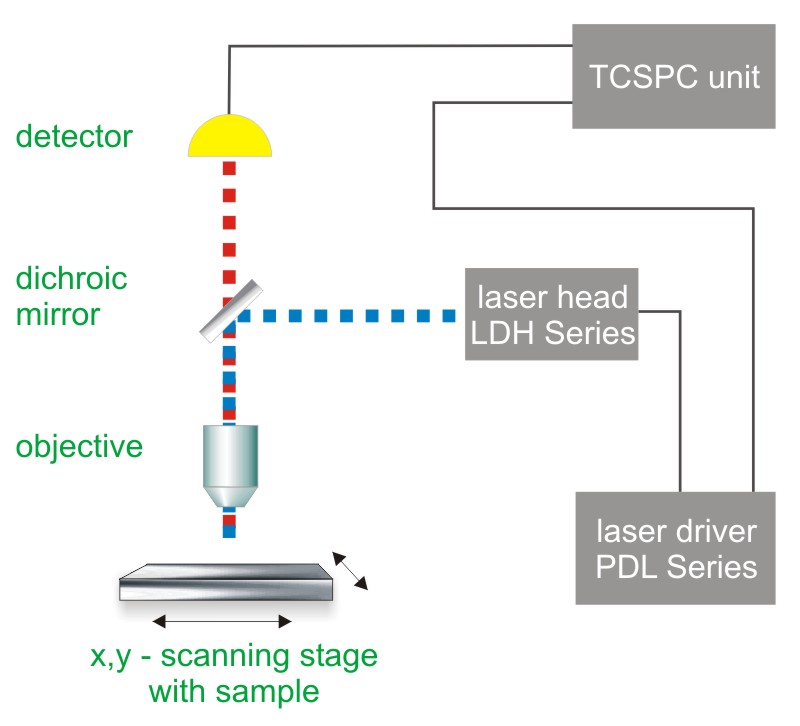
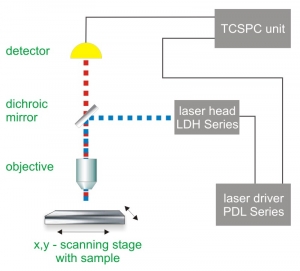 Time-Correlated Single Photon Counting (TCSPC) is used to determine the fluorescence lifetime. In TCSPC, one measures the time between sample excitation by a pulsed laser and the arrival of the emitted photon at the detector. TCSPC requires a defined “start”, provided by the electronics steering the laser pulse or a photodiode, and a defined “stop” signal, realized by detection with single-photon sensitive detectors (e.g. Single Photon Avalanche Diodes, SPADs). The measurement of this time delay is repeated many times to account for the statistical nature of the fluorophores emission. The delay times are sorted into a histogram that plots the occurrence of emission over time after the excitation pulse.
Time-Correlated Single Photon Counting (TCSPC) is used to determine the fluorescence lifetime. In TCSPC, one measures the time between sample excitation by a pulsed laser and the arrival of the emitted photon at the detector. TCSPC requires a defined “start”, provided by the electronics steering the laser pulse or a photodiode, and a defined “stop” signal, realized by detection with single-photon sensitive detectors (e.g. Single Photon Avalanche Diodes, SPADs). The measurement of this time delay is repeated many times to account for the statistical nature of the fluorophores emission. The delay times are sorted into a histogram that plots the occurrence of emission over time after the excitation pulse.
In order to acquire a fluorescence lifetime image, the photons have to be attributed to the different pixels, which is done by storing the absolute arrival times of the photons additionally to the relative arrival time in respect to the laser pulse. Line and frame marker signals from the scanner of the confocal microscope are additionally recorded in order to sort the time stream of photons into the different pixels.
Consequently the essential components of a FLIM set-up are:
- Pulsed laser source (diode lasers or multi-photon excitation)
- Single photon sensitive detector
- Dichroic mirror (to separate fluorescence signal from excitation light)
- Objective (to focus the excitation light into the sample and collect fluorescence signal)
- TCSPC unit to measure the time between excitation and fluorescence emission
Related technical and application notes:
- Application note: Fluorescence Lifetime Imaging (FLIM) in Confocal Microscopy
- Application note: Two-photon excitation using the MicroTime 200
- Technical note: FLIM NDD Upgrade Kit for the Olympus FluoView FV1000MPE
- Technical note: Time-Correlated Single Photon Counting (TCSPC)
- Technical note: Combination of MicroTime 200 with Asylum AFM
- Technical note: Combination of MicroTime 200 with Bruker AFM
- Technical note: Combination of MicroTime 200 with JPK AFM
- Technical note: FLIM: Scanning Speed vs. Excitation Rate
The following PicoQuant systems provides access to FLIM experiments and other lifetime measurements
Single Photon Counting Confocal Microscope
Luminosa combines state-of-the-art hardware with cutting edge software to deliver highest quality data while simplifying daily operation. The software includes context-based workflows for FLIM, FRET, and FCS, which allow the users to focus on their samples. Tight integration of hardware and software enable automated routines, e.g., for auto-alignment or laser power calibration, which increase ease of use as well as reproducibility of experiments. Still, an open mode of operation is available for full access to every optomechanical component via software.
Modular, time-resolved confocal fluorescence microscopy platform
The MicroTime 200 time-resolved fluorescence microscope system is a powerful instrument capable of Fluorecence Corelation Spectroscopy and its daughter techniques as well as Fluorescence Lifetime Imaging (FLIM) with single molecule detection sensitivity. It contains the complete optics and electronics for recording virtually all aspects of the fluorescence dynamics of microscopic samples or femtoliter volumes. The instrument gains its exceptional sensitivity and flexibility in combination with unprecedented ease-of-use from a unique fusion of miniaturized and highly sophisticated state-of-the-art technologies. Although, these technologies enable to run an instrument of comparable complexity and power without having to spend more time on instrument maintenance than on original scientific content, the MicroTime200 remains an open platform that allows the advanced scientist to easily built upon the open character of the instrument in order to realize highly customized applications
LSM Upgrade Kits from PicoQuant add FLIM capabilities to laser scanning microscopes from many major manufactures
Confocal Laser Scanning Microscopes (LSMs) are widely used tools in cell and molecular biology, biochemistry and other related sciences. PicoQuant's LSM Upgrade Kits greatly enhance the capabilities of these microscopes by extension to time-resolved techniques, and thereby providing not only Fluorescence Lifetime Imaging (FLIM) but also Fluorescence Correlation Spectroscopy (FCS) and a wealth of other time resolved techniques. The LSM Upgrade Kit combines PicoQuant products to a ready-to-use kit that fits your specific application on a state of the art Laser Scanning Microscope of your choice from Leica, Nikon, Olympus or Zeiss.
Upright time-resolved confocal microscope
The upright time-resolved microscope MicroTime 100 contains the complete optics and electronics for recording F(C)CS or FLCS data as well as fluorescence decays in small volumes by means of Time-Correlated Single Photon Counting (TCSPC). The system is based on a conventional upright microscope body that permist easy access to a wide range of sample shapes and sizes.
 The following core components are needed to build a system capable of FLIM measurements, which are (partly) available from PicoQuant:
The following core components are needed to build a system capable of FLIM measurements, which are (partly) available from PicoQuant:
- Laser drivers
- Laser or LED heads
- Photon counting detectors
- TCSPC electronics
- Analysis software
- Scanning device
- Galvo- or Piezo-scanner
- Confocal optics
Fluorescence Lifetime Imaging (FLIM) can be used for several applications such as:
- Local environment sensing
The fluorescence lifetime can change depending on the fluorophore environment (which means polarity, pH, temperature, ion concentration etc.) and is therefore used as a parameter for biological sensors. Specifically, fluorophores return to the ground state through radiative and non-radiative processes. Quenching of the excited state by external factors decreases the fluorescence lifetime. The resulting lifetime shortening provides information about the molecular environment of the fluorophore and even allows for quantitative distinction between subpopulations of quenched and unquenched fluorophores. - Detection of molecular interactions
A special case for the influence of the local environment on fluorescence lifetime is fluorescence resonance energy transfer (FRET), where the donor dye is quenched by the presence of an acceptor dye. Thus, a decrease of the fluorescence lifetime is indicative for FRET. In this way, the fluorescence lifetime serves as a sensor parameter for intra- and intermolecular interactions allowing for distance measurements in the nanometer range. - Detection of conformational changes
Applying an intramolecular labelling approach, the distance between the dye and the quencher or FRET acceptor can also vary along with different conformations of the labeled biomolecule. In this way, intramolecular changes e.g. due to folding or action of molecular motors are detectable. - Discrimination of multiple labels or background removal
With the advancement of fluorophores and microscopy, researchers now are able to use several fluorescent markers in parallel to assess different processes simultaneously. One challenge is that the employed fluorophores need to be distinguishable and have commonly used spectral characteristics. This limits the number of useful fluorescent markers. The analysis of the fluorescence lifetime can help to overcome these limitations. Additionally, the fluorescence lifetime enables to discriminate label fluorescence from the fluorescence background of the sample (e.g. cell or tissue) and thereby allows a higher detection efficiency and more accurate marker localization. - Tissue characterization by autofluorescence
The autofluorescence can be characteristic for a certain tissue and therefore be used e.g. for tumor detection. Typically, Two-Photon Excitation (TPE) is combined with Non-Descanned Detection (NDD) for deep tissue imaging, as these applications are generally more prevalent in tissues or organisms.
FLIM enables studying variations in Ca2+ levels in neurons and astroglia over time
![FLIM mapping of resting [Ca2+] in pyramid and astroglia loaded with OGB-1 including gap junction connected astrocytes.](/images/uploads/application_images/7289/example_ca2levels__500xp.png) Calcium ions (Ca2+) are an important messenger in brain signalling. In situ Monitoring of the resting concentration and rapidly changing concentrations of Ca2+ is important for understanding neural communication.
Calcium ions (Ca2+) are an important messenger in brain signalling. In situ Monitoring of the resting concentration and rapidly changing concentrations of Ca2+ is important for understanding neural communication.
The fluorescence lifetime of the dye Oregon Green BAPTA-1 (OGB-1) is highly sensitive to nanomolar [Ca2+], which makes it a great tool for mapping resting [Ca2+] in neurons and astroglia. Zheng et al. Published an improved technique that allows directly correlating the measured fluorescence lifetime to [Ca2+] values using a calibration.
FLIM provides several advantages over intensity-based measurements using ratiometric Ca2+ indicators: It is insensitive to emission intensity, fluctuations in dye concentration, focus drift, imaging depth, light scattering and photobleaching. Moreover, Zheng et al. showed that the fluorescence lifetime of OGB-1 does not depend on micro-viscosity, temperature, physiological changes in pH, as well as Mg2+ and Zn2+ concentrations. ![Example of [Ca2+] map (OGB-1 FLIM readout) in a spiny dendritic fragment, in resting conditions and ∼5 min after a short burst of back-propagating spikes. Color-coded scale applies for both images.](/images/uploads/application_images/7289/example_ca2levels_2__300px.png) Thus, OGB-1 is an excellent lifetime-based sensor for monitoring resting [Ca2+] in brain slices and in vivo.
Thus, OGB-1 is an excellent lifetime-based sensor for monitoring resting [Ca2+] in brain slices and in vivo.
In the first figure, a FLIM image of whole-cell OGB-1-loaded CA1 pyramidal cells and astroglia is shown. One can see that the CA1 pyramidal cell (dark blue) has a lower [Ca2+] than the astroglia (light blue). The two red shapes are pipette tips.
The second figure shows a spiny dendritic fragment of a CA1 pyramidal cell, in resting condition and about 5 min after a short burst of back-propagating action potentials. The FLIM reveals that the signalling activity led to a reduction of [Ca2+].
Set-up:
- LSM Upgrade Kit on Olympus FluoView FV1000
- Excitation: two-photon excitation, 80 MHz at 800 nm, Spectra Physics MaiTai
- TCSPC unit: PicoHarp 300
- Detector: PMA Hybrid 40
- Image processing: FIMAS in MATLAB
Images adapted and reproduced under CC BY license from Nature Publishing Group (Zheng et al., Neuron, Vol. 88, p. 277-288, 2015)
References:
Zheng et al., Neuron, Vol. 88, p. 277-288 (2015), DOI: 10.1016/j.neuron.2015.09.043
Zheng et al., Nature Protocols, Vol. 13, No. 3, p. 581-597 (2018), DOI: 10.1038/nprot.2017.154
Using fluorescence lifetime imaging microscopy to make invisible ink visible
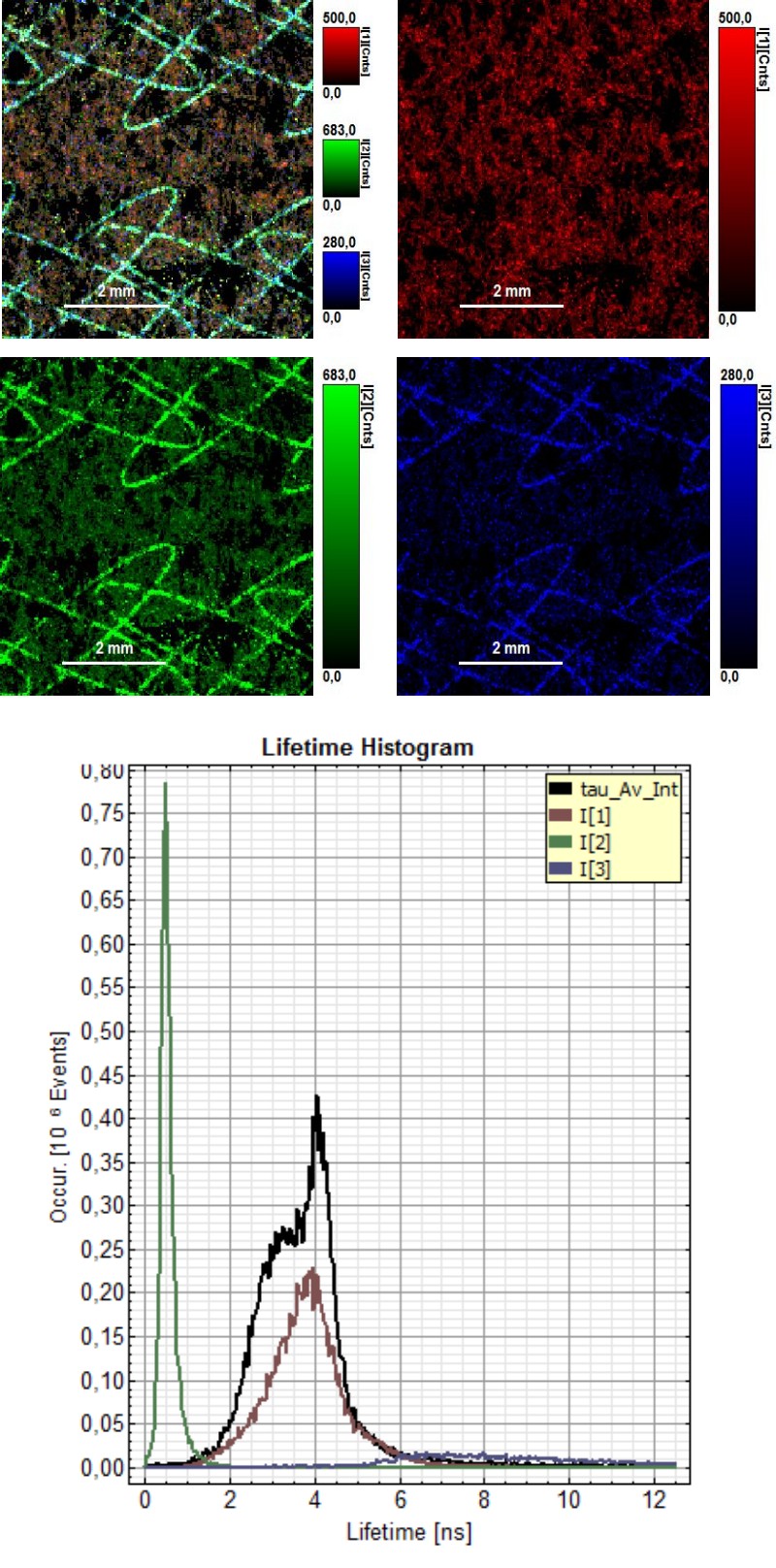 Invisible ink is a common security technology and authentication feature used for example in banknotes or passports. FLIM measurements can make invisible ink visible, due to the fluorescence lifetime of the used dye mixture. Therefore, it would be possible to identify the ink mixture and attribute it to the company producing that ink type.
Invisible ink is a common security technology and authentication feature used for example in banknotes or passports. FLIM measurements can make invisible ink visible, due to the fluorescence lifetime of the used dye mixture. Therefore, it would be possible to identify the ink mixture and attribute it to the company producing that ink type.
In this example, FLIM images of a paper showing a guilloché pattern written in invisible ink were acquired. The ink mixture consists in this case of two different dyes. To separate them, the FLIM data were fitted using a 3-exponential model. In the lifetime histogram, the shortest component of the fit corresponds to the paper auto fluorescence (0.5 ns), while the two longer lifetimes (3.6 ns and 6.6 ns) can be attributed to the two dyes forming the ink mixture.
The lifetime distribution can be visualized by performing a pixel-by-pixel fit and plotting the results as a Red/Green/Blue (RGB) color code. The shortest lifetime component (0.5 ns), corresponding to the paper auto fluorescence, is displayed in red. The lifetimes of the two dyes are shown in green (3.6 ns) and blue (6.6 ns). As can be seen from the lifetime histogram, the concentration of the dye with the longest lifetime was very low. Contributions from paper auto fluorescence can still be detected in the green and blue coded images. However, the paper contributions can be clearly distinguished from the ink dyes by applying a pattern matching analysis.
Furthermore, FLIM can be used to evaluate paper and printing quality and validate whether all components were added to the ink mixture.
Sharp edges and homogenous ink adsorption are an indication of high paper quality. Not all dots showed a uniform ink distribution and well-defined shape, as can be seen from the FLIM image, where the average lifetime is color coded. The lifetime histogram shows that the same three components as described above are present, but in a different ratio. In this case, the ink contained an equal amount of both dyes.
Set-up:
- MicroTime 100
- Excitation: 485 nm, 40 MHz
- Emission: 495 nm - 595 nm
- Olympus 50x/ 0.5 air objective
- Detection: PMA Hybrid
- Analysis: SymPhoTime 64
Samples courtesy of U-NICA Group, Switzerland.
Changes in fluorescence lifetimes help in visualizing humidity in beech wood
Control of humidity in wood is an important aspect for woodworking. The humidity of wood needs to be assessed and equilibrated before using it in further processing steps. For this purpose, FLIM measurements are a useful tool, as it can help to determine the humidity by monitoring the auto fluorescence of the wood. In the FastFLIM image below, the auto fluorescence lifetime clearly changes depending on the humidity. Dry wood has a longer lifetime (center) whereas moist wood has a shorter lifetime (right). The auto fluorescence lifetime of wood at room humidity lies in between these two states (left).
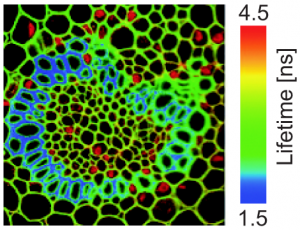
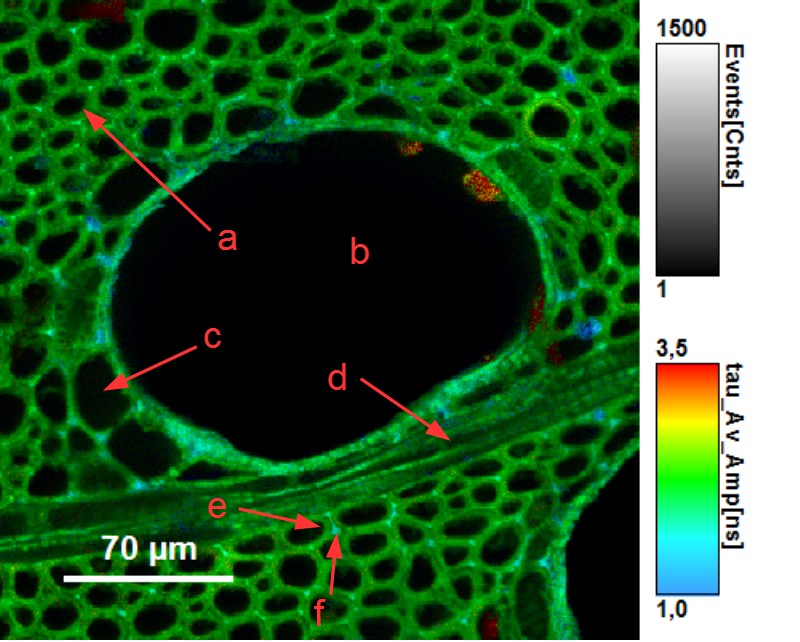 Beside assessing the humidity of wood, FLIM provides a detailed insight into the structure of sliced wood pieces. The picture below contains a horizontal cross section of ash wood (fraxinus excelsior) showing the different wood tissues: fiber cells (a), vessel cells (b), parenchym cell (c), wood ray (d). Fluorescence lifetime allows to distinguish between cell wall (e) and middle lamella (f).
Beside assessing the humidity of wood, FLIM provides a detailed insight into the structure of sliced wood pieces. The picture below contains a horizontal cross section of ash wood (fraxinus excelsior) showing the different wood tissues: fiber cells (a), vessel cells (b), parenchym cell (c), wood ray (d). Fluorescence lifetime allows to distinguish between cell wall (e) and middle lamella (f).
Set-up:
- MicroTime 100
- Excitation: 375 nm, 10 MHz
- Emission: 450 - 560 nm
- Analysis: SymPhoTime 64
Samples courtesy of Henry Bartsch, IHD Dresden, Germany.
Nanodiamonds as charge-sensitive fluorescent probes for lifetime imaging
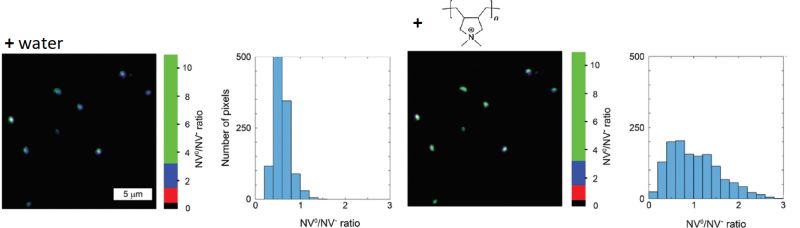
Nanodiamonds (ND) featuring fluorescent nitrogen vacancy (NV) centers have increasingly received attention as labels, as they are exceptionally photostable and do not blink. Since their fluorescence lifetime of approximately 17 ns is significantly longer than that of commonly used organic fluorophores, they provide great FLIM contrast. Furthermore, NDs are non-toxic and can thus be used for in vivo bioimaging studies.
The charge and emission properties of the NVs depends on the particles surface chemistry. In the negatively charged state (NV-), the emission occurs at 575 nm, while in the neutral state (NV0) light is emitted at 637 nm. Thus, the ND emission spectrum can report on changes to surface charge due to noncovalent binding events.
In this example, NDs were incubated first with water and then with the linear, positively charged polymer poly (diallyldimethylammonium chloride), which adsorbed to the ND surface. The NV0/NV- ratio was monitored via fluorescence intensity in two spectral detection channels. In order to suppress background noise, only pixels containing fluorescence with a lifetime longer than 13 ns were used for analysis. The observed change in NV0/NV- ratio indicates a change of ND surface charge after addition of the polymer.
Set-up:
- MicroTime 200
- Excitation: 532 nm, 1.2 mW power
- Objective: 60x water immersion (Olympus)
- Emission: BP 580/10 nm (Edmund Optics, OD4), BP 638/10 nm (Edmund Optics, OD4)
- Image processing: MATLAB (Mathworks)
Courtesy of Vladimira Petrakova, Czech Technical University in Prague, Czech Republic
Reference: Petrakova et al., Nanoscale, Vol. 7, p. 12307-12311 (2015), DOI: 10.1039/c5nr00712g
Characterizing fluorescent nitrogen vacancies in irradiated diamond nano crystals by means of Fluorescence Lifetime Imaging (FLIM)
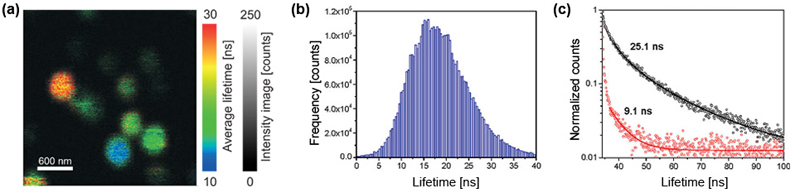
Luminescent single-nanometer-sized diamond-like carbon particles have received considerable attention in biophysics, material science, nano-medicine, and photonics. Partly this is related to their extraordinary material properties, such as chemical inertness, biocompatibility, and hardness, and also partly due to their exceptional photostability. All of these applications desire the availability of sub-10 nm particles that show bright and stable photoluminescence.
The fluorescence lifetime image of NV centers in nanodiamonds shows lifetimes with a mean value of around 17 ns, only slightly longer than the bulk value of the defects. In order to gain a statistically significant insight into the distribution of fluorescence lifetimes, a FLIM image of several nano crystals on a glass surface was recorded (Fig. a). The suggested reason for the fluorescence lifetime distribution (Fig. b) is the dipole orientation of the NV centers with respect to the glass coverslip they are deposited on. Typical fluorescence lifetime decay curves of two different nanodiamonds are shown in Fig.c.
Set-up:
- home-built scanning confocal microscope with integrated AFM (MFP-3D Asylum Research)
- TCSPC electronics: PicoHarp 300
- Analysis: SymPhoTime
Courtesy of Jörg Wrachtrup, University of Stuttgart
Reference: Tisler et al., ACS Nano, Vol.03, p.1959-1965 (2009)
Fluorscent probes enable monitoring of chloride concentration in insect organs
Example for exploiting the fluorophore lifetime dependence on the local environment
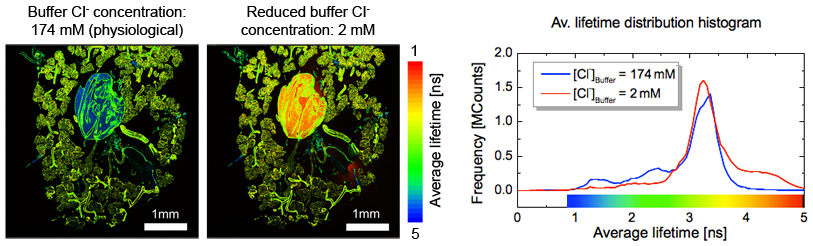
Cockroach glands are a well-established model system for studying epithelial ion transport. Salivary glands of the american cockroach were stained with the Cl--sensitive dye MQAE. Thus after calibration, Cl--concentration can be determined as well as the response time to various stimuli like different buffer concentrations wherein the salivary glands are embedded. Recording FLIM images with physiological Cl--concentration and reduced Cl--concentration allows the mapping of Cl--concentration to measured fluorescence lifetime and thereby enabling the mapping of the Cl--concentration throughout the salivary system. The two FLIM images show the fluorescence lifetime in the salivary gland at physiological (left) and low (right) Cl- concentrations. The central salivary reservoir displays a significant change in chloride concentration (blue → red). The lifetime difference in the surrounding salivary glands is much less pronounced (green). This can also be seen in the fluorescence lifetime distribution histogram.
- MicroTime 200
- Excitation wavelength 780 nm (TPE), SpectraPhysics MaiTai
- Detection: 420 nm -460 nm
- Analysis: SymPhoTime
Courtesy of Carsten Hille, Carsten Dosche, Potsdam University
Determining lipid order by means of fluorescence lifetime
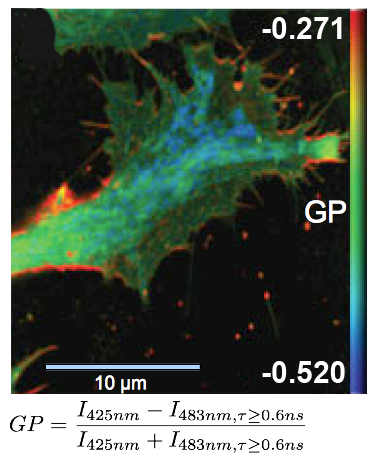
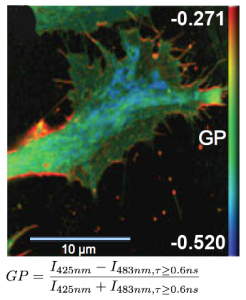 FLIM measurements facilitate the differentiation between ordered and disordered membrane phases. The membrane dyes Laurdan and di-4-ANEPPDHQ can be used to image membrane order due to a spectral blue-shift in the fluorescence emission as well as a lifetime shift between the liquid-ordered and liquid-disordered phases. These images typically take the form of a normalized intensity ratio image known as a generalized polarization (GP) plot. Here, the known excited state photo physics is exploited via Time-Correlated Single-Photon Counting (TCSPC) to demonstrate GP contrast enhancement for these two probes. The image shows a GP plot of a Laurdan stained, fixed BAEC cell that combines lifetime and spectral changes. The plasma membrane at the cell surface shows higher order (red) compared to the inner cell compartments (blue).
FLIM measurements facilitate the differentiation between ordered and disordered membrane phases. The membrane dyes Laurdan and di-4-ANEPPDHQ can be used to image membrane order due to a spectral blue-shift in the fluorescence emission as well as a lifetime shift between the liquid-ordered and liquid-disordered phases. These images typically take the form of a normalized intensity ratio image known as a generalized polarization (GP) plot. Here, the known excited state photo physics is exploited via Time-Correlated Single-Photon Counting (TCSPC) to demonstrate GP contrast enhancement for these two probes. The image shows a GP plot of a Laurdan stained, fixed BAEC cell that combines lifetime and spectral changes. The plasma membrane at the cell surface shows higher order (red) compared to the inner cell compartments (blue).
Set-up:
- combined MicroTime 200 and LSM Upgrade Kit (Leica TCS SP5)
- Excitation: two-photon excitation at 800 nm, SpectraPhysics MaiTai
- Analysis: SymPhoTime
Courtesy of Katharina Gaus, Centre for Vascular Research, University of New South Wales, Sydney, Australia
Reference: Owen et al., Microsc. Res. Tech. 73(6), (2010)
Using fluorescence lifetime information to disciriminate between multiple fluorophores and for background removal
Due to the increasing number of fluorescent proteins and specific labeling techniques, it has become possible to stain multiple target molecules in parallel and observe their interactions as well as their multiple localizations within the sample. This raises the question about how to discriminate between the various labels. In the classical way using a spectral approach, the multiple components in such a system are stained each with spectrally different fluorophores allowing for multicolor imaging. Spectral unmixing helps to raise the number of distinguishable labels. This number is even increased by including the fluorescence lifetime as an additional parameter. Consequently, more fluorophores can be monitored and separated simultaneously within a sample, and the specific labels can be efficiently separated from inherent autofluorescence.
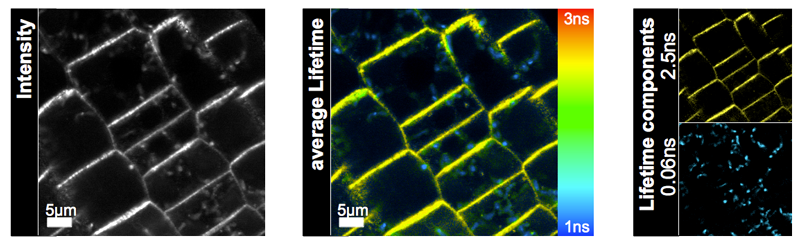
This example from plant biology shows Arabidopsis root cells transformed with a GFP-tagged PIN2 variant. The PIN-protein group is involved in root graviotology. Pin2-GFP excited with a pulsed 470 nm laser has a significant longer lifetime than the autofluorescence of the Arabidopsis root cells (middle panel “average Lifetime”). A three-component lifetime fit was applied and the 2.5 ns component was specific for GFP fluorescence (upper image in “Lifetime components”), whereas the shorter lifetime species belonged to background fluorescence (lower image in “Lifetime components”).
Set-up:
- MicroTime 200
- Excitation: 470 nm
- Emission: 500 nm - 540 nm
- Data Processing: SymPhoTime
Courtesy of Alexander Dovzhenko, Klaus Palme, Freiburg University
What is fluorescence and fluorescence lifetime?
Many species such as molecules, complexes, or nanoparticles can emit photons after absorbing light in a process called fluorescence. The fluorescence lifetime is an intrinsic characteristic of such a light emitting species and it indicates how long it will remain in an electronically excited state before returning to the ground state. Each emitting species has a characteristic luminescence lifetime that can be influenced by its environment.
What is Fluorescence Lifetime Imaging (FLIM)?
Fluorescence Lifetime Imaging (FLIM) produces an image based on the differences in excited state decay rates. Thus, FLIM is a fluorescence imaging technique where the contrast is based on the lifetime of individual fluorophores rather than their emission spectra.
How does Fluorescence Lifetime Imaging (FLIM) work?
A FLIM image consists of a 2D grid of pixels that divide the sample into small areas of identical size. For each pixel, the fluorescence lifetime is determined by means of Time-Correlated Single Photon Counting (TCSPC). This requires positioning the microscope’s excitation laser at each spot and collect the arrival times of emission photons for a certain time period before moving to the next spot. The arrival times for each pixel are sorted into a historgram, from which the fluorescence lifetimes can be extracted via mathematical means.
Why include fluorescence lifetime information when imaging?
By including the fluorescence lifetime, you not only gain an additional image contrast, but also additional information that would not be accessible through intensity imaging alone. For example: being able to sense the local environment, detecting molecular interactions or conformational changes, discriminate between multiple spectrally similar lavels, or effective removal of background emission.
Why use the picosecond timescale for Fluorescence Lifetime Imaging (FLIM)?
Fluorescence usually occurs on the scale of a few nanoseconds. So using picosecond pulsed lasers for excitation and Time-Correlated Single Photon Counting units with picosecond time bins is an excellent choice for studying these processes with a great temporal resolution.
What is the advantage of FLIM over intensity imaging?
As fluorescence lifetime does not depend on concentration, sample absorption or thickness, photo-bleaching and/or excitation intensity, it is more robust than intensity based methods. At the same time, the fluorescence lifetime is affected by a range of environmental parameters (i.e. pH, ion or oxygen concentration, molecular binding, etc), which enables functional imaging of many kinds.
Which scientific fields can benefit from FLIM?
Over the last decade, FLIM has become a standard method for studying proteins, organelles, cells or tissues in life science and biology. The method can also provide deeper insights into the excited state dynamics and structure-emission relationship in many other materials and is being used more and more in fields such as materials science and semiconductor testing.
Latest 10 publications related to FLIM
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this application and our products in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.

 Contact us
Contact us Luminosa
Luminosa MicroTime 200
MicroTime 200 LSM Upgrade Kit
LSM Upgrade Kit MicroTime 100
MicroTime 100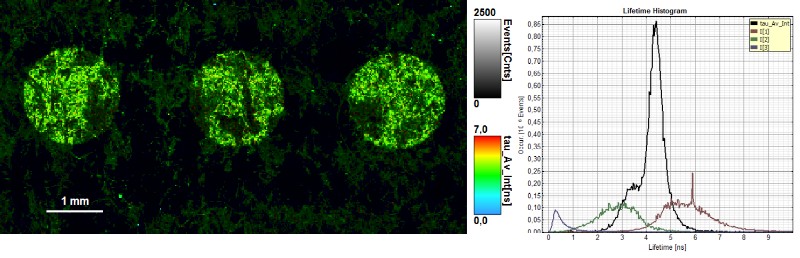






![Example of [Ca2+] map (OGB-1 FLIM readout) in a spiny dendritic fragment, in resting conditions and ∼5 min after a short burst of back-propagating spikes. Color-coded scale applies for both images.](https://www.picoquant.com/images/uploads/application_images/7289/example_ca2levels_2.png)
![FLIM mapping of resting [Ca2+] in pyramid and astroglia loaded with OGB-1 including gap junction connected astrocytes.](https://www.picoquant.com/images/uploads/application_images/7289/example_ca2levels.png)
