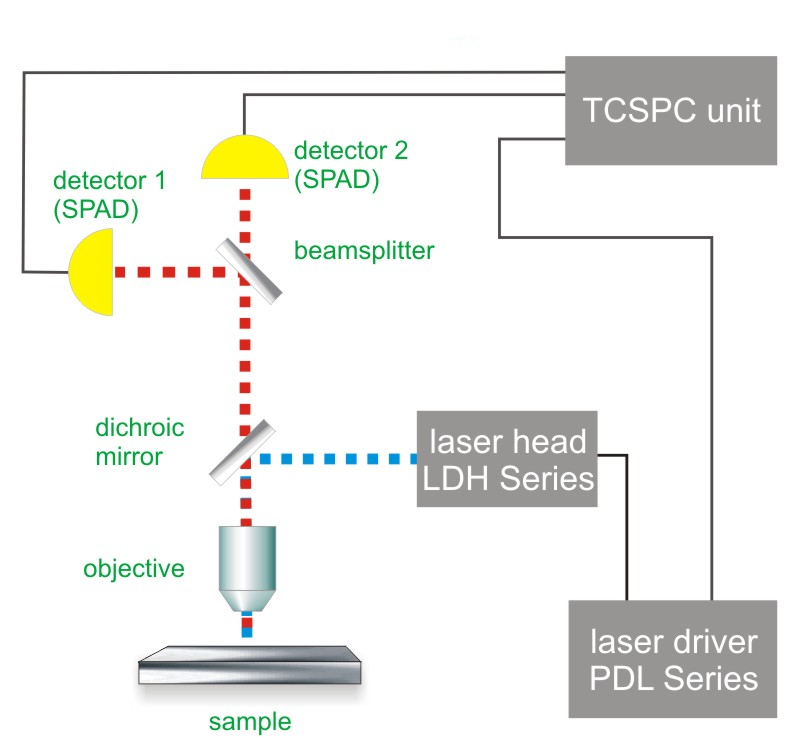
 Since FCS-based-techniques measure fluctuations caused by single molecules entering or leaving the observation volume (or changing their fluorescence properties), a highly sensitive confocal microscope is a prerequisite for FCS experiments.
Since FCS-based-techniques measure fluctuations caused by single molecules entering or leaving the observation volume (or changing their fluorescence properties), a highly sensitive confocal microscope is a prerequisite for FCS experiments.
For a simple FCS experiment, a single laser and a single photon counting detector is needed. For FCCS, typically two lasers and two detectors are employed.
Because FLCS builds on the additional information purveyed by the fluorescence decay characteristics, Time-Correlated Single Photon Counting (TCSPC) and its corresponding requirements like pulse lasers are necessary for FLCS experiments.
Consequently, the essential components of a FCS set-up are:
- laser source(s) (pulsed for FLCS)
- single photon sensitive detector(s)
- dichroic mirror (to separate fluorescence signal from excitation light)
- water objective with high NA to reduce abberation and increase quantum yield.
- photon counting card for FCS
- for FLCS: TCSPC unit to measure the time between excitation and fluorescence emission
- FCS acquisition and analysis software
Related technical and application notes:
The following PicoQuant systems provide access to FCS experiments
 Luminosa
Luminosa
Single Photon Counting Confocal Microscope
Luminosa combines state-of-the-art hardware with cutting edge software to deliver highest quality data while simplifying daily operation. The software includes context-based workflows for FLIM, FRET, and FCS, which allow the users to focus on their samples. Tight integration of hardware and software enable automated routines, e.g., for auto-alignment or laser power calibration, which increase ease of use as well as reproducibility of experiments. Still, an open mode of operation is available for full access to every optomechanical component via software.
 MicroTime 200
MicroTime 200
Modular, time-resolved confocal fluorescence microscopy platform
The MicroTime 200 time-resolved fluorescence microscope system is a powerful instrument capable of Fluorecence Corelation Spectroscopy and its daughter techniques as well as Fluorescence Lifetime Imaging (FLIM) with single molecule detection sensitivity. It contains the complete optics and electronics for recording virtually all aspects of the fluorescence dynamics of microscopic samples or femtoliter volumes. The instrument gains its exceptional sensitivity and flexibility in combination with unprecedented ease-of-use from a unique fusion of miniaturized and highly sophisticated state-of-the-art technologies. Although, these technologies enable to run an instrument of comparable complexity and power without having to spend more time on instrument maintenance than on original scientific content, the MicroTime200 remains an open platform that allows the advanced scientist to easily built upon the open character of the instrument in order to realize highly customized applications
 LSM Upgrade Kit
LSM Upgrade Kit
LSM Upgrade Kits from PicoQuant add time-resolved capabilities to laser scanning microscopes from many major manufactures
Confocal Laser Scanning Microscopes (LSMs) are widely used tools in cell and molecular biology, biochemistry and other related sciences. PicoQuant's LSM Upgrade Kits greatly enhance the capabilities of these microscopes by extension to time-resolved techniques, and thereby providing not only Fluorescence Lifetime Imaging (FLIM) but also Fluorescence Correlation Spectroscopy (FCS) and a wealth of other time resolved techniques. The LSM Upgrade Kit combines PicoQuant products to a ready-to-use kit that fits your specific application on a state of the art Laser Scanning Microscope of your choice from Leica, Nikon, Olympus or Zeiss.
 MicroTime 100
MicroTime 100
Upright time-resolved confocal microscope
The upright time-resolved microscope MicroTime 100 contains the complete optics and electronics for recording F(C)CS or FLCS data as well as fluorescence decays in small volumes by means of Time-Correlated Single Photon Counting (TCSPC). The system is based on a conventional upright microscope body that permist easy access to a wide range of sample shapes and sizes.
 The following core components are needed to build a system capable of FCS measurements, which are (partly) available from PicoQuant:
The following core components are needed to build a system capable of FCS measurements, which are (partly) available from PicoQuant:
- Laser drivers
- Laser or LED heads
- Photon Counting Detector
- TCSPC Electronics
- Analysis software
- Confocal optics
Along with an appropriate model, FCS can be used to obtain quantitative information, such as
- Average absolute concentrations
- Diffusion coefficients
- Hydrodynamic radii
- Kinetic chemical reaction rates
- Singlet-triplet dynamics
- Binding ratios (FCCS)
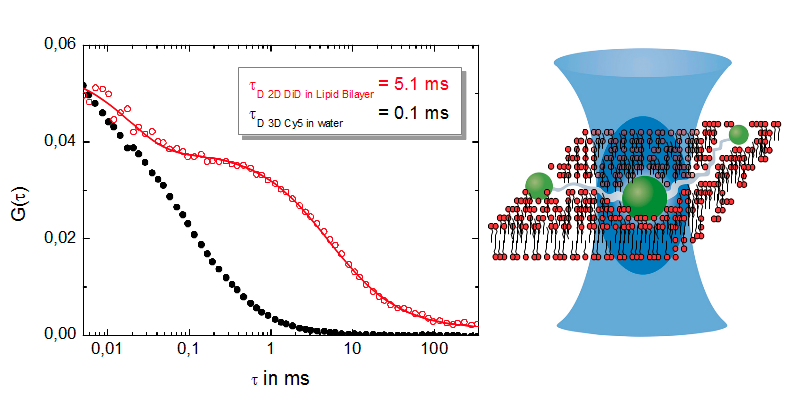
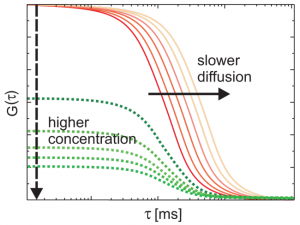
FCS allows to measure diffusion rates that are typically expressed as the diffusion coefficient of the molecule or molecular complex. FCS is even capable to detect and distinguish between single and multiple component diffusion.
Furthermore, this technique enables the investigation of lateral and rotational diffusion of fluorophores as well as conformational dynamics providing e.g. information about hydrodynamic radii and singlet-triplet dynamics.
In addition, FCS provides a direct and independent, calibration-free measure of molecular concentration within a sample. Based on the concentration measurement and cross-correlation between two fluorophores (dual-color FCCS), data are used to detect molecular association and dissociation and to determine the stoichiometry of molecular complexes. In this way, it is possible to determine kinetic rate constants, i.e. on and off kinetics of complex formation, as well as enzyme dynamics and intramolecular dynamics in vitro and in living cells.
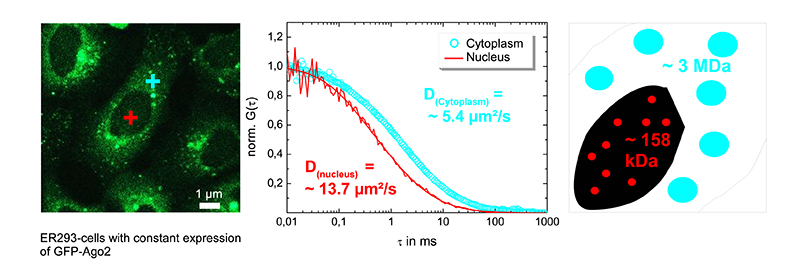
Using FCS to distinguish between different complexes in cellular sub compartments
Ago2, an argonaut protein, is part of the RNA-Induced Silencing Complex (RISC). Understanding the localization and assembly mechanism of RISC helps to unveil the regulation of gene expression. The mobility of GFP-Ago2 was measured in ER293 cells in the nucleus (red cross in left figure) and the cytoplasm (blue cross in left figure). The corrsponding FCS curves are shown in the middle figure.
Measuring the diffusion coefficient inside the nucleus (about 13.7 µm/cm2) and in the cytosol (approx. 5.4 µm/cm2) revealed that RISCs complexes differ in their diffusion behaviour, which was confirmed to be due to size differences of nuclear and cytosolic RISCs (right figure).
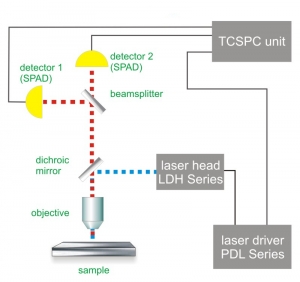
Set-up:
Courtesy of M. Gärtner, P. Schwille, TU Dresden, Germany
Reference: Ohrt et al., Nucl. Acids Res. 2008
Performing Fluorescence Correlations spectroscopy (FCS) in the deep UV: proof of principle
Many molecules ranging from small aromatics to large biomolecules like proteins can be detected by their intrinsic fluorescence in the deep UV as they contain appropriate chromophoric groups such as tryptophan and tyrosine.
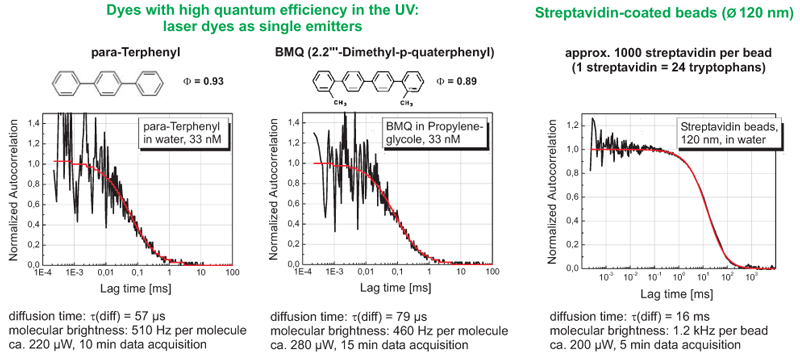
266 nm excitation grants access to the intrinsic fluorescence of tryptophan-containing proteins. This proof of principle experiment demonstrates that with the specially adapted MicroTime 200, FCS measurements are possible even in the UV. Different laser dyes and for comparison streptavidin coated beads were excited at 266 nm. Fluorescence was detected at wavelengths above 300 nm.
Set-up:
Latest 10 publications related to FCS
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this application and our products in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.

 Contact us
Contact us
 Luminosa
Luminosa MicroTime 200
MicroTime 200 LSM Upgrade Kit
LSM Upgrade Kit MicroTime 100
MicroTime 100