Fluorescence Microscopes
Luminosa
Luminosa
Remarkably simple.Precisely yours.
Single Photon Counting Confocal Microscope
Explore new paths in confocal microscopy
Luminosa is an all-in-one fluorescence microscope that pairs highest data quality with remarkably simple day-to-day operation. It easily integrates into any researcher’s toolbox and becomes a time-efficient, reliable companion for scientists starting to explore the use of time-resolved fluorescence methodologies as well as for experts wanting to push the limits. Truly a microscopy system that everybody can rely on.
You want to learn more?
Download brochure >
Download datasheet >
Watch our product presentation >
Get a quote and ask your questions >
Latest additions:
- Add-ons that enhance spatial resolution, enable metabolic imaging, or extend live cell experiments
- NovaFLIM software for fast analysis of FLIM, FLIM-FRET, and anisotropy data
- NovaISM software for FLIM analysis with high resolution and contrast
- New workflow for FRAP and a Python interface LumiPy. Software is ready for Download
- LumiPy is now available on GitHub
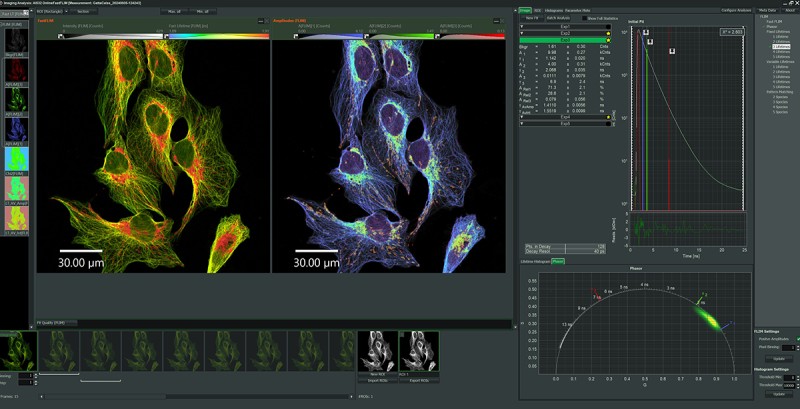
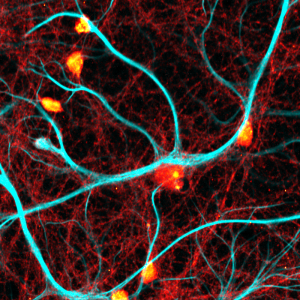
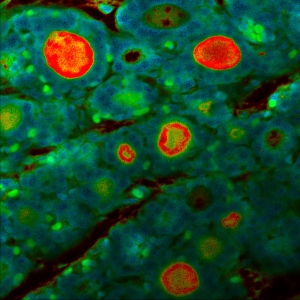
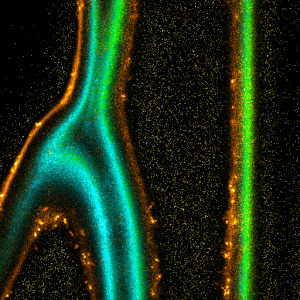
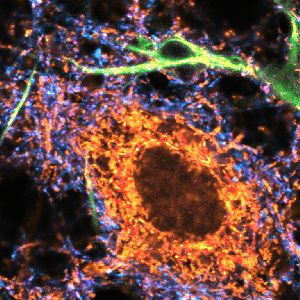
Impressions of Luminosa's capabilities
Remarkably simple. Precisely yours.
PicoQuant has acquired 25 years of experience with time-resolved single photon counting. During this time, PicoQuant has also cultivated strong ties to the scientific community, which we value immensely. We have gathered the extensive knowledge both of our scientific staff and expert scientists, and merged it to create our best time-resolved instrument & software package so far: Luminosa.
QUALITY AND PRECISION YOU CAN TRUST
Optimal performance for single molecule investigations in every measurement context: One-click auto-alignment procedure even without requiring a sample. Galvo scanning (maximum speed) and objective scanning (maximum photon detection efficiency) on the same microscope.
SAVE TIME AND SIMPLY FOCUS ON YOUR SAMPLES
Context-based, intuitive workflows guide you to efficiently harness the full power of smFRET, FCS and FLIM with confidence. Get analysis results with minimal user interaction. GPU-based algorithms provide fast and reliable results.
ADVANCED FLEXIBILITY
Adjust the observation volume to match the dynamics of your FCS and smFRET assays with a single click. An open mode of operation is available for full access to every optomechanical component via software.
What customers say about Luminosa
Luminosa Advancing Bioimaging Core Facilities
Insights from Prof. Marco Fritzsche
 Prof. Marco Fritzsche, Scientific Director of the Oxford-ZEISS Centre of Excellence (Oxford-ZEISS CoE), highlights how Luminosa marks a major step forward for research in biophysics and immunology. Its user-friendly design enables even inexperienced users to generate meaningful data, while offering sophisticated features for experts.
Prof. Marco Fritzsche, Scientific Director of the Oxford-ZEISS Centre of Excellence (Oxford-ZEISS CoE), highlights how Luminosa marks a major step forward for research in biophysics and immunology. Its user-friendly design enables even inexperienced users to generate meaningful data, while offering sophisticated features for experts.
Download application note on label-free metabolic imaging of tissue samples >
Benefit from our highly qualified support team
You will profit from the combined expertise of our support team. All supporters hold a PhD degree in the natural sciences. Tap into their years of experience to advance your research!
OUR SUPPORT TEAM OFFERS
- initial training with hardware and software during system installation, either on-site or remote
- follow-up training on demand, tailored to your needs
- application counseling regarding questions from sample preparation and measurement setting optimization to data analysis
- troubleshooting in case of microscope hardware and software problems
- system repairs
DEMONSTRATIONS AND TEST MEASUREMENTS
The team also performs instrument demonstrations and test measurements with your samples in our application lab in Berlin. If you want to find out what Luminosa can reveal about your samples, request a session.
Request demo
What customers say about our support
 Scientific guidance and user training
Scientific guidance and user training
Researchers wishing an in-depth introduction to the principles of time-resolved fluorescence microscopy and its applications to the Life Sciences can attend PicoQuant's annual 3-day course including lectures and hands-on training.
Applications
Dynamic structural biology at the single molecule level
Many protein structures have been solved successfully using methods such as X-ray crystallography or cryo-EM. However, intrinsically disordered proteins or proteins which do not crystallize pose serious problems for these techniques. Moreover, the observation of dynamic conformational changes of proteins as they are performing their tasks in their native environment calls for different approaches.
Here, single molecule fluorescence approaches such as FRET and FCS open new possibilities, as they enable the study of individual molecules at room temperature in solution or even in cells, with the potential to discriminate subpopulations in different locations.
smFRET monitors distance changes between two or more fluorescent labels attached to different protein residues, which provides valuable information about conformational changes, folding or protein interactions. This complements protein structural models and molecular dynamics simulations, allowing researchers to develop a more complete understanding of protein function.
FCS on the nanosecond timescale (nsFCS) reveals the reconfiguration time of the protein chains of intrinsically disordered or folding proteins. Combining this information with polymer physics theory and molecular simulations yields a clearer picture of disordered protein dynamics.
FCS on the microsecond to second timescale measures molecular diffusion behavior, which depending on the experimental context can shed light on the protein mobility, its microenvironment, or on protein oligomerization and interaction.
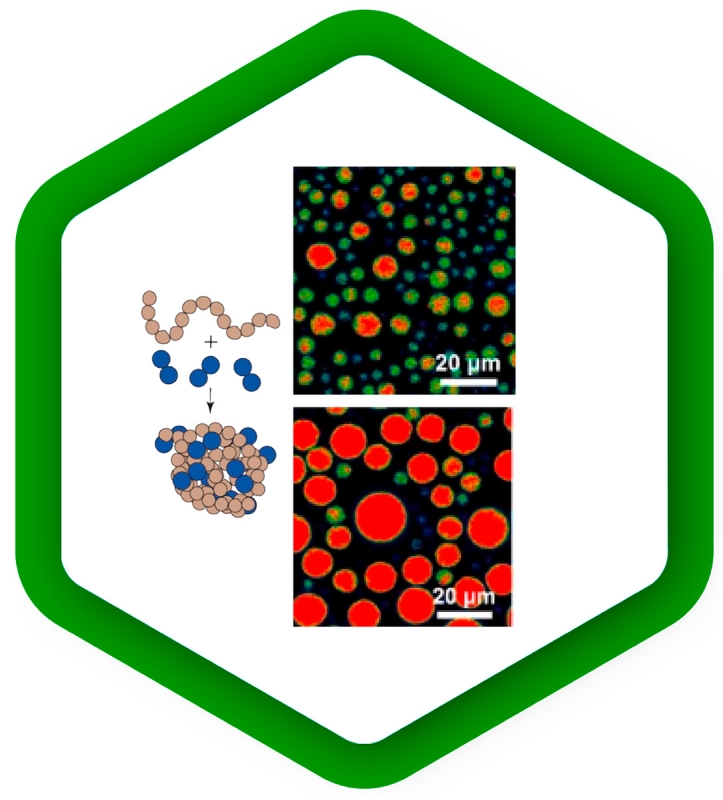
Cellular mechanisms driven by phase separation
Liquid-liquid phase separation is emerging as a key mechanism for regulating the spatial organization of biomolecules in cells. It underlies compartmentalization by condensation of subsets of molecules, which promotes protein interactions. As phase separation is a very dynamic process, it responds quickly to changes in environmental conditions.
Fluorescence methods can be used to study the kinetics of phase separation, to follow the distribution of molecules into different phases, to observe the dynamics of separate phases, and to probe their different properties:
FCS measures the concentration of a labeled species; its extensions FCCS and FLCS can even measure the concentrations of several species simultaneously. Moreover, FCS reports on the diffusion rate of the molecules under study, which can differ between phases.
Fluorescence anisotropy experiments nicely complement FCS by observing the rotational motion of molecules, which depends on the viscosity or rigidity of the local environment.
FLIM of fluorescent sensors can read out environmental parameters such as pH, temperature, membrane tension, or the concentration of various ions in real time. This additional dimension of information can help to interpret the dynamics of different phases and understand their properties from a physical point of view.
Application note:
To read about investigations of synapsin-1 liquid-liquid phase separation with Luminosa using FLIM and multi-point FCS, check our application note.
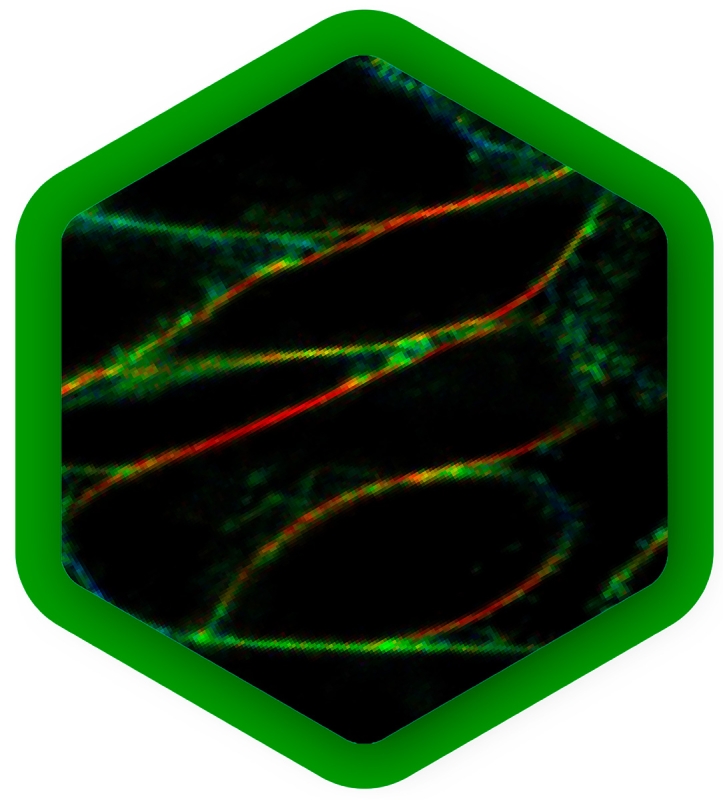
Environmental sensing
The properties of a protein's native environment inside a cell, such as molecular crowding, pH fluctuations, or plasma membrane alterations, influence its function. But in many cases, not all of these parameters are known, so they cannot accurately be reproduced in experiments.
In the last few years, a range of fluorescent sensors has been developed that change their fluorescence lifetime in response to a specific environmental change, for example temperature, calcium concentration, glucose concentration or membrane tension. With FLIM, these parameters can be read out quantitatively in real time from live cells in a non-invasive manner, providing context to better understand protein function or regulation.
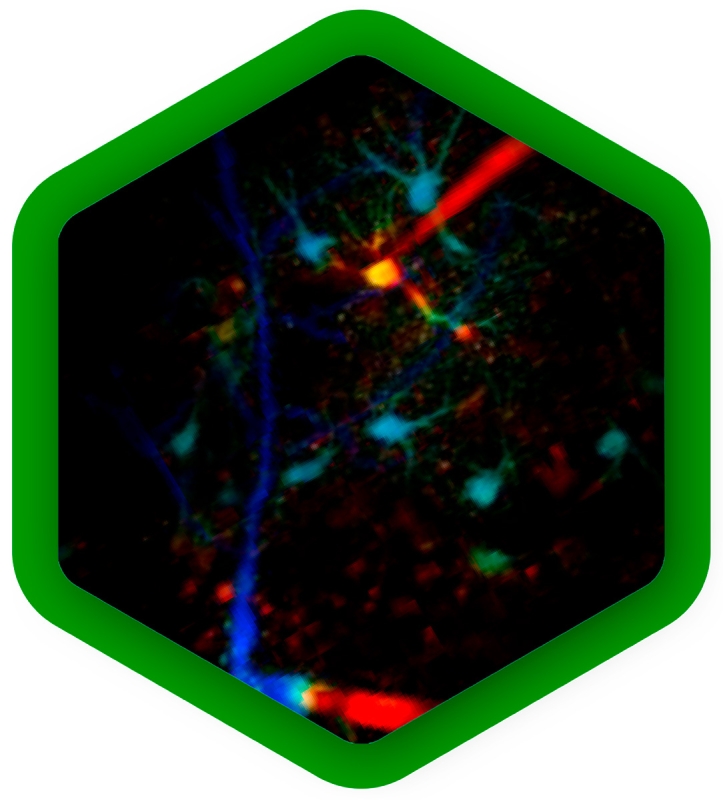
Mapping dynamics and structure of cellular membranes
Cellular membranes are highly complex and dynamic structures. Their molecular composition of lipids and membrane proteins is precisely tuned to obtain the physical and chemical properties necessary for their physiological function. Complementary fluorescence methods can be combined to approach biological membranes from different angels:
FCS of fluorescently labeled lipids or membrane proteins yields diffusion rates and protein mobility. It also reports concentrations of the molecules under study, which can vary across locations.
With anisotropy experiments one obtains the molecular orientation, and changes thereof. Thus, anisotropy imaging can visualize ordered and disordered membrane phases, or monitor the rotational motion of molecules.
FLIM of environmental sensor fluorophores can quantify important physical parameters, for example membrane tension or lipid order.
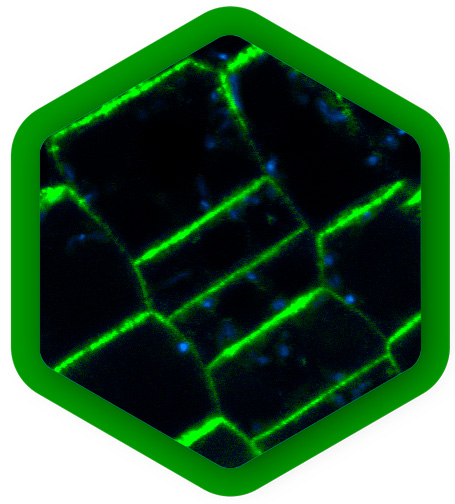
Plant development and structure
The study of plants is critical for addressing a wide range of global challenges, from food security and environmental sustainability to medicine and climate change. It also contributes to numerous industrial applications such as agricultural improvement, wood-based products, extraction of medical compounds, or biofuel production.
Many plant components, such as chlorophyll in leaves, or lignin and flavonoids in woody cell walls, show strong autofluorescence. These signals can be used for label-free imaging of a plant`s physiological state, it`s morphology, or biomaterial characterization, for example. Here, FLIM can provide additional lifetime contrast for distinguishing components, complementary to spectral contrast.
Autofluorescence is often considered problematic when it spectrally overlaps with fluorescence staining. Also in this case, signal from fluorescent labels that has a different fluorescence lifetime pattern can easily be separated from autofluorescent background with FLIM.
Live imaging of plants offers new insights for example into tissue patterning, cell specification and division. At the cellular level, FLIM-FRET can determine protein localization, dynamics and quantify interactions. FCS is a complementary method that also quantifies protein movements and concentrations and detects oligomerization.
Core Methodologies
Fluorescence Lifetime Imaging (FLIM)

FLIM is a fluorescence imaging technique that resolves and displays the lifetimes of individual fluorophores rather than their emission spectra. The fluorescence lifetime is defined as the average time that a molecule remains in an excited state prior to returning to the ground state by emitting a photon. Since fluorescence lifetime is unrelated to concentration, absorption by the sample, sample thickness, photo-bleaching and/or excitation intensity, FLIM is less prone to artifacts than intensity based methods.
Benefits of FLIM:
- can be used to distinguish between fluorophores
- provides an additional dimension of information
- complementary to spectral information
- technique of choice for many kinds of functional imaging, as a fluorophore’s lifetime can be influenced by environmental parameters such as pH, ion or oxygen concentration, or molecular binding
Read in this article that appeared in Microscopy Today (January 2023) how Luminosa simplifies FLIM: https://doi.org/10.1093/mictod/qaac006
FLIM-FRET – lifetime-based Förster resonance energy transfer

FRET is a non-radiative process whereby energy from an excited fluorescent molecule (donor) is transferred to a nearby non-excited fluorophore (acceptor) in the range of 2-10 nm. The energy transfer results in donor quenching and leads to changes in the fluorescence intensity of the two fluorophores and a shortening of only the donor lifetime.
In contrast to standard FRET where one measures changes in fluorescence intensity, lifetime-based FRET enables quantitative analysis by using the fluorescence lifetime of the donor molecule as a probe, which is independent on concentration over a broad range. This is crucial since in biological systems like cells the fluorophore concentration often cannot be accurately determined and compared amongst different cells.
Benefits of FLIM-FRET: lifetime-based FRET can identify different sub populations with different FRET efficiencies, where intensity-based FRET would only detect the average value
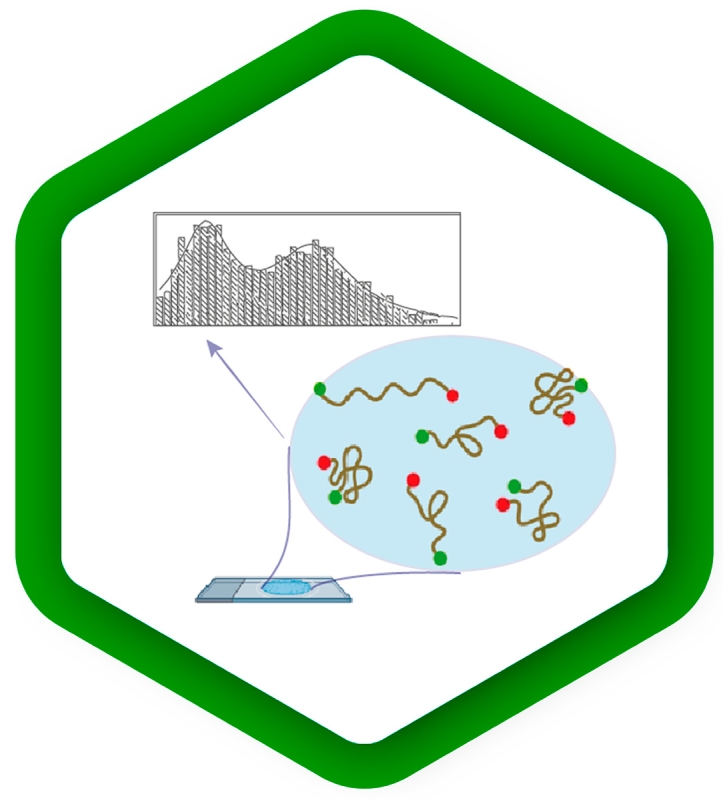
smFRET – single-molecule Förster resonance energy transfer

In this type of experiments, the FRET process is observed within a single molecule (bearing a donor and acceptor), or between interacting partners that are either freely diffusing through the confocal volume immobilized on a surface.
A technique which can be quite helpful for smFRET is Pulsed Interleaved Excitation (PIE), where several pulsed lasers are synchronized. The laser pulses are separated on a nanosecond time scale to allow simultaneous recording of the temporal behaviour of a sample molecule.
In an smFRET experiment, this allows exciting the donor and acceptor alternately. In this way, the acceptor dye is excited independently of the FRET process to confirm its presence and photoactivity. Molecules lacking an active donor or acceptor are separated from active FRET complexes. This makes it possible to differentiate a FRET molecule, even with a very low FRET efficiency, from a molecule with an absent or non-fluorescing acceptor.
Read about streamlined smFRET experiments in this article that appeared in PhotonicsViews (February/March 2023): https://doi.org/10.1002/phvs.202300002
Fluorescence Correlation Spectroscopy (FCS)

Fluorescence Correlation Spectroscopy (FCS) is a correlation analysis of temporal fluctuations of the fluorescence intensity. It offers insights into the photophysics that cause these characteristic fluctuations as well as into the diffusion behavior and absolute concentrations of detected particles. FCS enables the determination of important biochemical parameters such as the concentration and size or shape of the particle (molecule) or viscosity of their environment.
Fluorescence Lifetime Correlation Spectroscopy (FLCS)
Fluorescence Lifetime Correlation Spectroscopy (FLCS), is a method that uses picosecond time-resolved fluorescence detection for separating different FCS contributions.
FLCS is of particular advantage when using spectrally inseperable fluorophores that differ in their lifetime for Fluorescence Cross-Correlation Spectroscopy (FCCS) because it offers elimination of spectral cross talk and background. It also offers a way around detector afterpulsing artifacts.
Fluorescence Cross-Correlation Spectroscopy (FCCS)
Fluorescence Cross-Correlation Spectroscopy (FCCS) discriminates FCS signals from two different species based on their different emission spectra.
Anisotropy imaging

Measurement of steady-state and particularly time-resolved fluorescence anisotropy offers fascinating possibilities to study molecular orientation and mobility as well as processes that affect them. In general, anisotropy does not depend on the concentration of fluorophores, i.e. is independent of detected signal intensity. This is of particular importance for the interpretation of smFRET measurements where fluorophore mobility might affect the resonance transfer efficiency. Time-resolved anisotropy measurements are more informative, as steady-state ones only report time averaged values, without direct insight into the dynamics of the process.
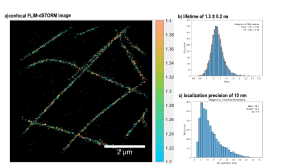
Customized mode modified for super-resolved FLIM with PAINT and dSTORM

Luminosa offers a customized mode for method development, in which every optomechanical component is fully accessible via software and parameters can be chosen freely.
This mode was exploited to go beyond standard FLIM and demonstrate super-resolved FLIM. On the one hand, FLIM-PAINT imaging was performed in cooperation with the research group of Guillermo P. Acuna from the University of Fribourg and on the other hand, FLIM-dSTORM imaging was done in cooperation with the group of Jörg Enderlein from the University of Göttingen.
Single-molecule localization microscopy (SMLM) provides images with a spatial resolution beyond the diffraction limit by sequentially locating individual molecules and subsequently assembling a super-resolved image. There are different mechanisms for switching molecules between a bright and a dark state to allow localization of only a small subset per image frame. One is DNA Point Accumulation for Imaging in Nanoscale Topography (DNA-PAINT), which uses short fluorescently labeled oligonucleotides that bind transiently to complementary, target-bound DNA molecules. Another possibility is direct Stochastic Optical Reconstruction Microscopy (dSTORM), where fluorophores blink, i.e. are switched on stochastically, emit, and quickly revert to the dark state.
In confocal imaging the scanning area, number of pixels and pixel dwell time together define the acquisition time per image. For PAINT, this needs to match the binding kinetics of the DNA-PAINT imager strands, such that the acquisition time is significantly smaller than the average binding time. For dSTORM, the acquisition time should be tuned according to the blinking kinetics of the fluorophore.
The resulting confocal FLIM image series was analyzed as it is usually done for SMLM measurements to obtain a final super-resolved image. Lastly, the photon arrival times of each single-molecule event were retrieved and analyzed to get lifetime contrast.
Benefits of FLIM-SMLM:
- spatial super-resolution due to PAINT or dSTORM
- confocal sectioning ability
- additional lifetime contrast due to FLIM, for multiplexing or FRET
- use conventional commercially available microscope with single excitation laser
- hold-focus option for long measurement times available
Read the original publication to learn more about FLIM-PAINT: https://doi.org/10.1002/smtd.202201565
System Highlights
New software concepts increase ease-of-use and reliability
- Fast results with minimal user interaction thanks to GPU accelerated algorithms
- Context-based workflows for FCS, FLIM, and single molecule detection
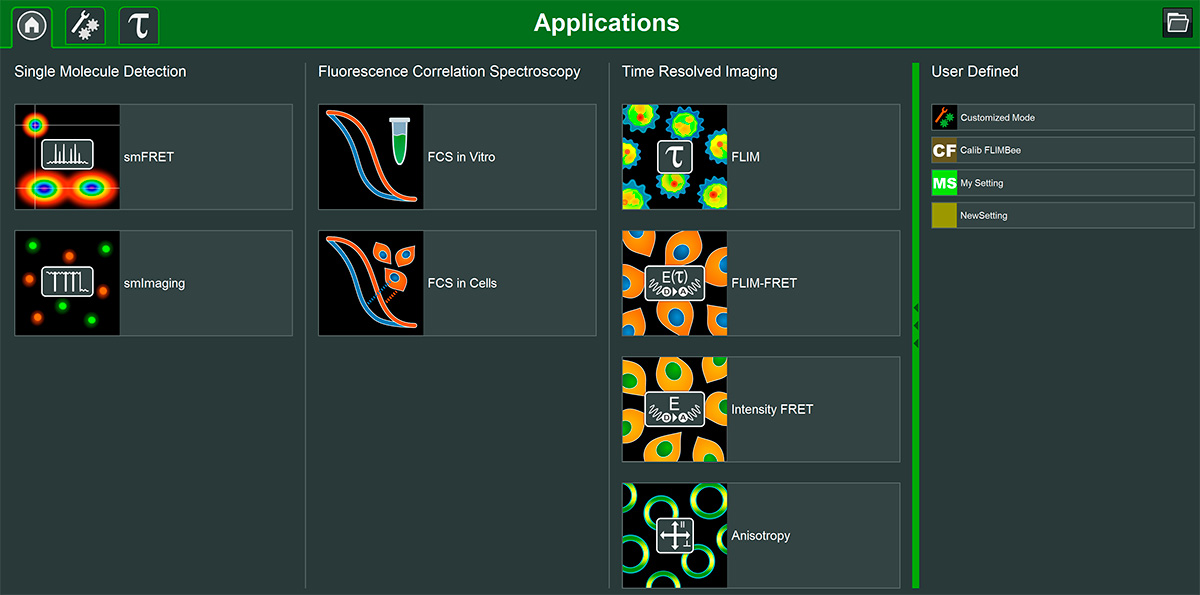
The Luminosa software includes single molecule detection, FCS, and time-resolved imaging methods, as well as the possibility to flexibly define custom measurement and analysis modes. Its design permits quick and easy workflows for experiments, which allow the users to focus on their samples. Clearly arranged interfaces showing only parameters relevant for each application promote reproducible measurements yielding consistent datasets.
Simply select the fluorophores in use, and the software automatically configures excitation lasers, the beampath and the detection. It takes care of parameters important for time-resolved measurements, such as laser repetition rate, and pulsed interleaved excitation. Several online previews of the data during acquisition enable the users to instantly check the sample and data quality, saving precious instrument time. Users can explore their data immediately thanks to fast GPU-based analysis routines.
Software feature highlights
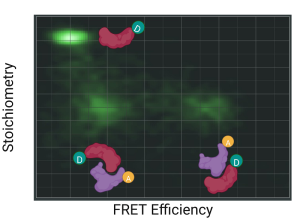
FRETcompass
- Online preview of efficiency vs. stoichiometry histogram
- Automated determination of correction factors used in single molecule FRET experiments
- Based on the community benchmark study published in 2018
- Fluorescence lifetime decays of donor only, low FRET and high FRET populations in all channels
LumiFinder
- Automated detection and measurement of immobilized emitters
- For single molecule studies, particularly single molecule FRET measurements
- Localization of immobilized emitters according to adjustable selection criteria
- Point measurements at identified locations, with definable duraction
- Online preview of data acquired at each point
Enhanced integration of hardware and software enables new functionality
Imaging large samples
- Spiral scan: create an overview in DIC, epi-illumination, or transmission mode covering large areas of several mm² in your samples in just a few seconds.
- Reference map: for further measurements at selected regions or points of interest, their spatial relationships saved for analysis
- Tiling and stitching of rectangular areas
- Hold focus feature
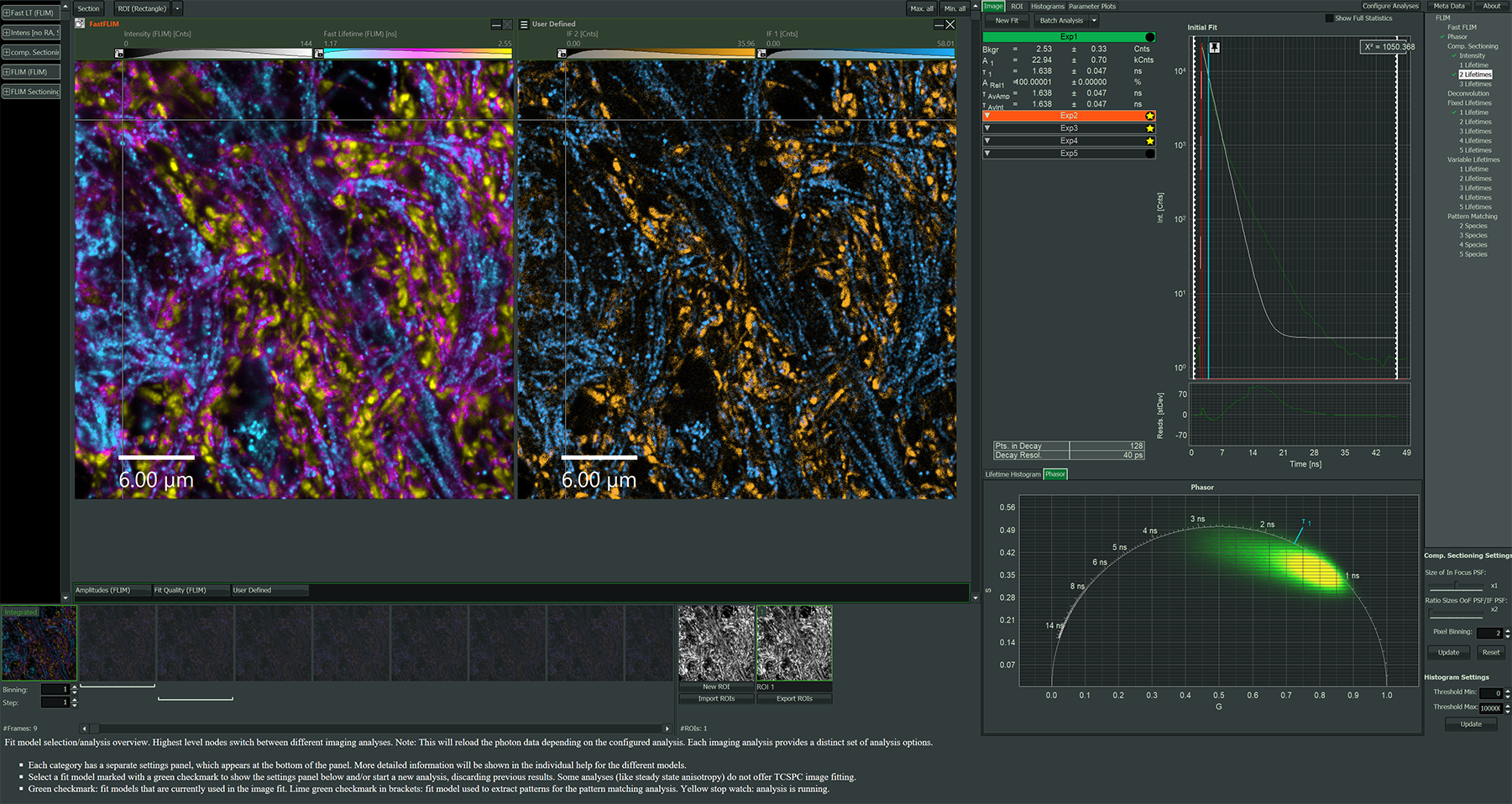
Software packages for advanced FLIM analysis
NovaFLIM
- Fast analysis of FLIM, FLIM-FRET, and anisotropy data, based on GPU-accelerated algorithms
- Efficient batch analysis of z-stacks, time-lapse series and stitched images
- Advanced, flexible ROI handling
- Reproducible ROI selection incorporating histograms of fitted parameters
NovaISM
- Powerful software to combine Image Scanning Microscopy (ISM) and FLIM analysis
- Higher resolution than confocal imaging
- State-of-the-art computational sectioning in combination with lifetime species separation, improving contrast and resolution
- Seamless integration with Luminosa
- Comprehensive data export options
Cutting-edge hardware
- Confocal system based on an inverted microscope
- Versatile excitation system with laser wavelengths from 375 to 1064 nm
- Scanning options: FLIMBee galvo scanner and piezo objective scanning (speed for imaging or highest sensitivity for single molecule studies)
- Up to 6 truly parallel detection channels with SPAD and/or Hybrid PMTs
- < 700 ps dead time per channel and 5 ps time bins
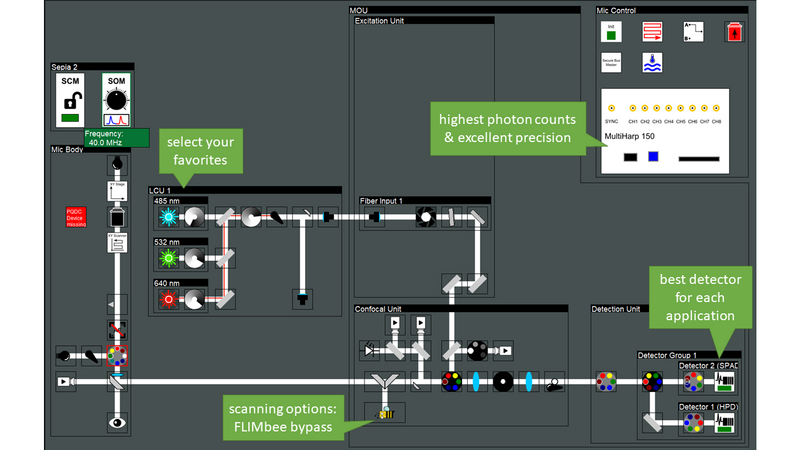
Luminosa has been designed for best performance in the lifetime domain. Moreover, the microscope is simultaneously optimized for single photon sensitivity, enabling experiments at the single molecule level. Experience with PicoQuant`s existing confocal MicroTime 200 system informed the optical design of the new microscope, and guided the selection of the best hardware components. The optical design is optimized for highest sensitivity by incorporating only the minimal number of optical elements in the best quality. Furthermore, the user can choose a galvo scanner for high speed or bypass it with the click of a button and use a piezo objective scanner instead. This option is not available on other confocal microscopes. Piezo scanning prevents loss of signal at the scanning mirrors and thus yields approximately 20 to 30 % more photons depending on the wavelength.
Luminosa incorporates the latest generation of PMA Hybrid detectors and TCSPC electronics for time-resolved measurements and rapidFLIMHiRes. Capitalizing on the ultra-short dead time of the MultiHarp TCSPC modules of only 650 ps, sustained photon count rates of up to ~ 78 Mcps can be achieved. With this, one can capture up to 15 FLIM frames per second, depending on galvo scanner speed, image size and sample brightness.
Add-ons
Several add-ons are available for Luminosa that expand the systems capabilties towards enhanced spatial resolution, metabolic imaging, or extended live cell experiments.
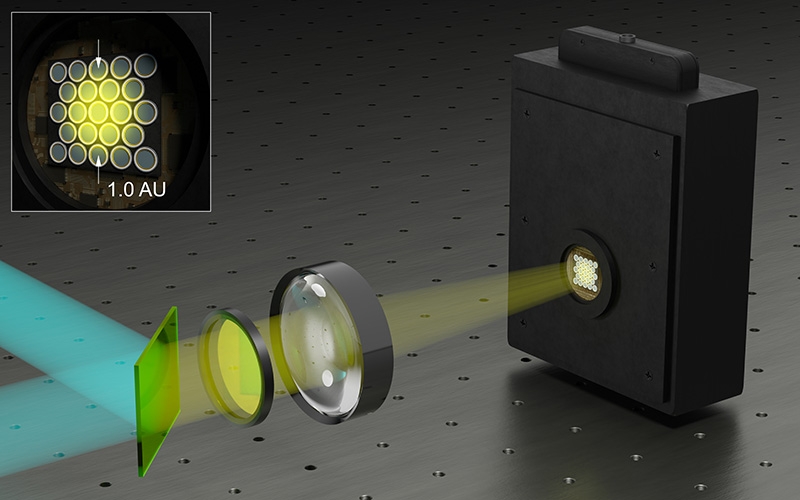
PDA-23 Detection: Confocal time-resolved detection with a SPAD array
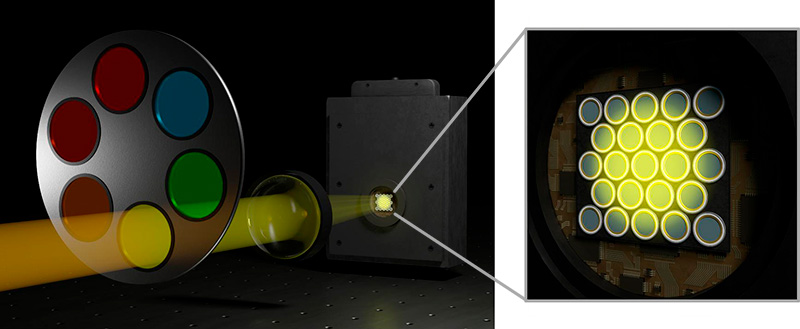
The SPAD array module PDA-23 is not just an addition to the Luminosa microscope. It is a transformative upgrade that redefines the boundaries of time-resolved confocal fluorescence microscopy.
Functional imaging with more resolution and higher contrast
Combine the resolution enhancement and increased contrast of Image Scanning Microscopy (ISM) with the functional information of FLIM.
Seamless integration
Designed exclusively for Luminosa, ensuring perfect compatibility, optimized performance and robust daily handling including streamlined auto-alignment. In combination with NovaISM advanced analysis software, the user can effciently move from acquisition to pixel reassignment, FLIM analysis, computational sectioning, and deconvolution.
Customizable research tool
Leverage the open hardware and (.ptu) data format to create and adapt new time resolved methodologies for your research goals with confidence built upon 25 years of experience in time resolved photon detection. Explore what the SPAD array can bring from imaging to fluctuation analysis and single molecule fluorescence modalities.
Precision meets compatibility
The PDA-23 detector is perfectly matched with our MultiHarp 160 TCSPC module.
PDA-23:
- Large effective fill factor
- High photon detection efficiency
- Timing resolution below 100 ps
- Low dark count rates
MultiHarp 160:
- Up to 64 independent input channels
- 5 ps timing resolution
- Ultrashort dead time of 650 ps, no dead time across channels
Experience the synergy of ISM and FLIM
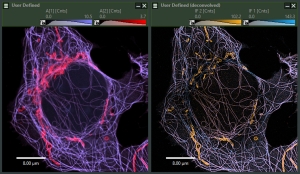
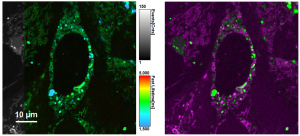
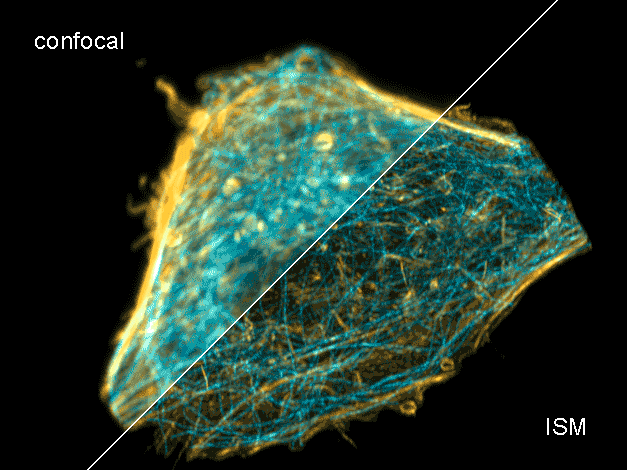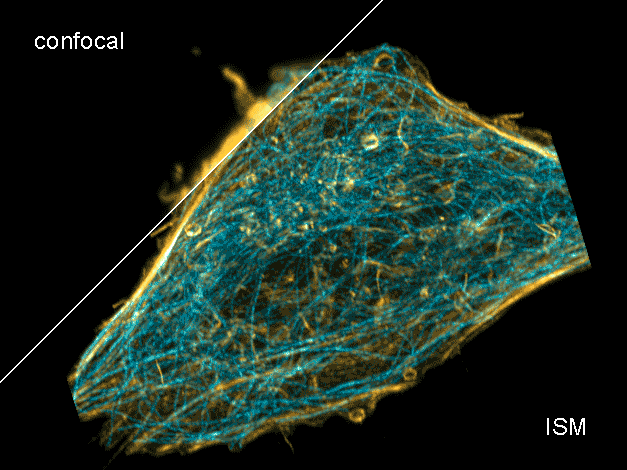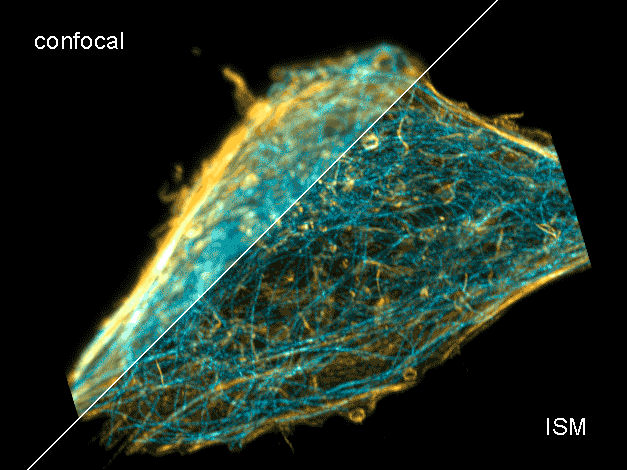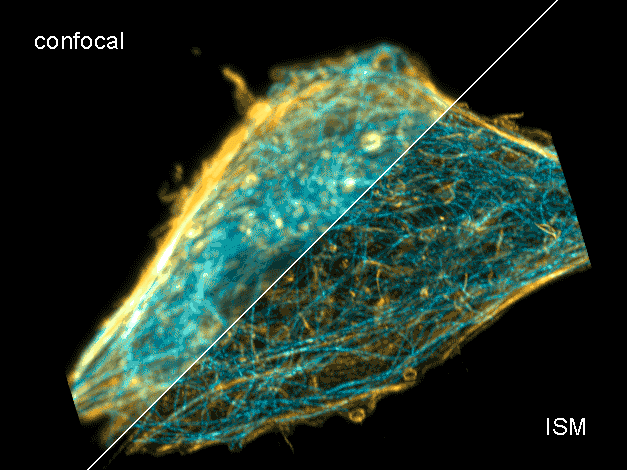 Combine the enhanced spatial resolution of Image Scanning Microscopy (ISM) and a big gain in contrast by rejecting out-of-focus light with the rich functional information and multiplexing possibilities of FLIM. This synergy allows for a deeper exploration of cellular dynamics and molecular interactions, providing a level of detail that was previously unattainable in a conventional confocal system.
Combine the enhanced spatial resolution of Image Scanning Microscopy (ISM) and a big gain in contrast by rejecting out-of-focus light with the rich functional information and multiplexing possibilities of FLIM. This synergy allows for a deeper exploration of cellular dynamics and molecular interactions, providing a level of detail that was previously unattainable in a conventional confocal system.
Truly simultaneous acquisition on all channels
Uniquely to Luminosa, both the PDA-23 detector and the other point confocal detectors can be used in parallel, to allow e.g. multiplexing of high-resolution and reference channels.
ISM benefits
- Each array element approximates zero-size confocal pinhole
- Increased optical sectioning
- Resolution increase of about 30 % after pixel re-assignment
- For 485 nm excitation: resolution down to 155 nm FWHM after pixel re-assignment and 120 nm FWHM after deconvolution
- Computational sectioning for improved contrast
Luminosa software features for ISM-FLIM
- Online pixel re-assignment
- Shift vectors automatically determined
- Online lifetime contrast
- FLIM analysis for marker multiplexing
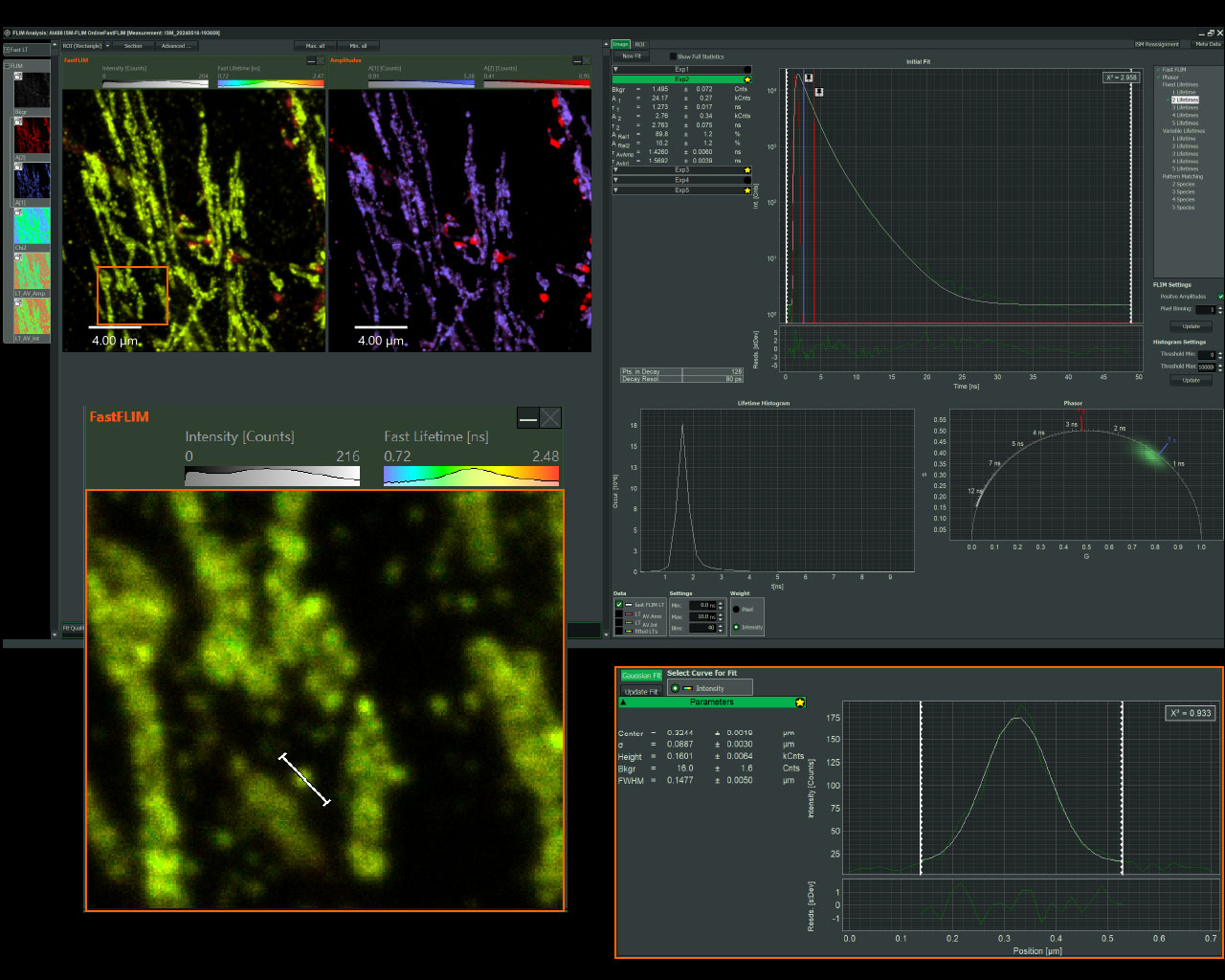
Sample courtesy of Rizzoli group, Department of Neuro- and Sensory Physiology, University of Göttingen Medical Center.
For questions or a quote contact us >
Easy-to-use, integrated fs laser for metabolic imaging
 Benefits
Benefits
- For monitoring metabolic imaging of NAD(H) activity with FLIM
- Add-on includes:
- fs fiber laser from TOPTICA at 780 nm
- Optics for coupling into Luminosa and controlling laser power
- Software integration into customized workflows
For questions or a quote contact us >
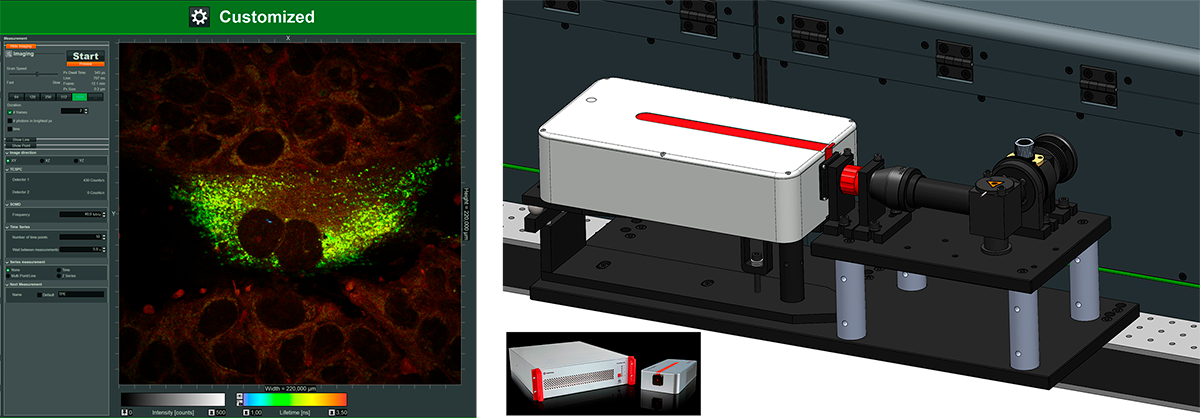
Exit port: Coupling of any external detection-unit, for even more options
Benefits
- For coupling of external detectors, spectrographs
- Add-on includes:
- Fiber-coupling or free-space-coupling optics
- Software integration into customized workflows
- Can be used truly parallel with the internal detectors
- Exemplary use cases
- Add a spectrograph with a single molecule sensitive CCD camera
- Add an extra detector unit with tunable filters like FlexLambda
- Add a Superconducting nanowire single-photon detector
For questions or a quote contact us >
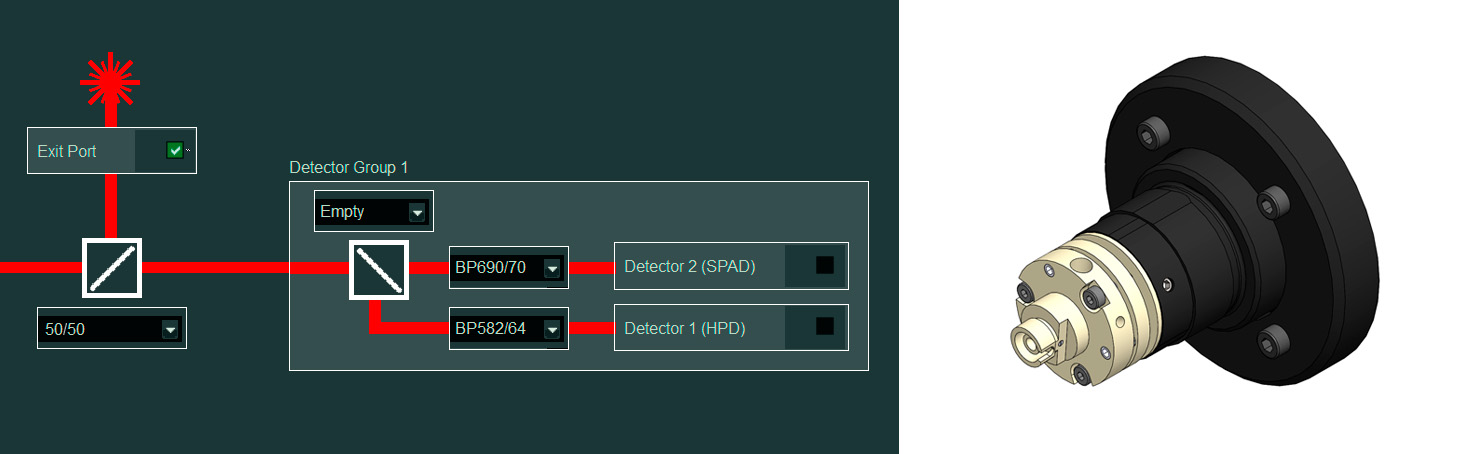
Stage top incubator: Extended live cell experiments

Benefits
- From Tokai Hit, including:
- Sample chamber + objective lens heater + temperature and CO2 controller
- Sample holders + lids for well plates, dishes, slides, and more
- Feedback regulation (optional)
- Software (not integrated with Luminosa software)
For questions or a quote contact us >
Next Events with Luminosa
We will demonstrate Luminosa at the following events. Contact us for options to meet our experts there.
Biophysical Society Annual Meeting 2026
February 21 - 25, 2026 | San Francisco, USA
The Biophysical Society annual meetings are the largest annual gathering of biophysicists around the world. The meetings include symposia, workshops, 15 subgroup programs, over 500 platform speakers selected from submitted abstracts, the Biophysical Society Lecture, more than 4,000 packed poster presentations, as well as educational exhibits, exhibitor presentations, and career development sessions.
PicoQuant Student Award:
PicoQuant confers the Student Award in the Biological Fluorescence Subgroup. This award is given to an outstanding Master's or PhD student who has recently made a significant contribution to our research community. The prize consists of a 750 USD honorarium and an invitation to present a 15-minute research talk at the Biological Fluorescence Subgroup Meeting during the Biophysical Society Annual Meeting.
Our product highlights:
- Single Photon Counting Confocal Microscope Luminosa
- Event Timer & TCSPC Unit PicoHarp 330
- 1-Color Gain-Switched Picosecond Laser Unico
- 3-Color Gain-Switched Picosecond Laser Prima
- Hybrid Photomultiplier Detector Assembly PMA Hybrid Series
- Compact FLIM and FCS Upgrade Kit for Laser Scanning Microscopes LSM Upgrade Kit
Our posters:
A Python Interface for Feedback Adaptive Workflows on the Commercial Microscope Luminosa
Presentation date: 23.02.2026 - 1:45 pm
Location: Exibith Halls ABC - Nr. B407
Poster session: Single-Molecule Spectroscopy
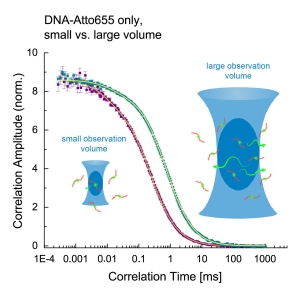
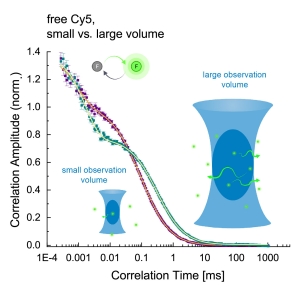
SPAD Array Detection Eneables Calibration-Free FCS for the Mesurement of Absolute Diffusion Coefficents and Concentrations
Presentation date: 23.02.2026 - 1:45 pm
Location: Exibith Halls ABC - Nr. B402
Poster session: Single-Molecule Spectroscopy
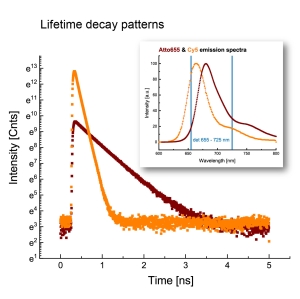
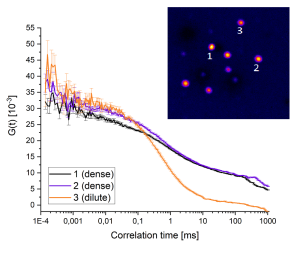
Combining ISM and FLIM for High-Fiedelity Lifetime Imaging in Dense Environments
Presentation date: 24.02.2026 - 1:45 pm
Location: Exibith Halls ABC - Nr. B434
Poster session: Machine Learning, Artificial Intelligence, and Bioinformatics II
Meet us at booth 514.
Anniversary Workshop on "Single Molecule Spectroscopy and Super-resolution Microscopy"
September 23 - 26, 2025 | Berlin, Germany
Since 1995, PicoQuant organizes the annual Workshop on “Single Molecule Spectroscopy and Super-resolution Microscopy” which brings together the top researchers in the field. The event provides an interdisciplinary platform for exchanging ideas and recent results between researchers and professionals working in physics, chemistry, biology, life, and materials science.


 Contact us
Contact us