Fluorescence Spectrometers
FluoTime 300
High-End Photoluminescence Spectrometer
- Steady-state and time-resolved (TCSPC, MCS) operation mode
- Highly modular and flexible design, optimal for upgradability
- Fully automated for lifetime ranges from picoseconds to seconds
- Double or single monochromator in excitation and emission with the unique feature of switching between additive and subtractive in emission
- Superior sensitivity with > 32 000:1 water Raman SNR
- Intuitive acquisition and analysis EasyTau 2 software
Upgrades:
- Micro-photoluminescence upgrade for combining microscopy and spectroscopy
- Integrating sphere for absolute luminescence quantum yield measurement
- Versatile sample holders for handling liquids and solids
- NEW: Class 1 laser safety
 The FluoTime 300 is a high performance fluorescence spectrometer for materials science, life science, and photochemistry applications. Our fully automated setup contains complete optics and electronics for recording steady-state spectra as well as fluorescence decays by means of Time-Correlated Single Photon Counting (TCSPC) or Multichannel Scaling (MCS) from a few picoseconds to several seconds. The FluoTime 300 provides superior sensitivity, spectral, and temporal resolution that allow the acquisition and analysis of
The FluoTime 300 is a high performance fluorescence spectrometer for materials science, life science, and photochemistry applications. Our fully automated setup contains complete optics and electronics for recording steady-state spectra as well as fluorescence decays by means of Time-Correlated Single Photon Counting (TCSPC) or Multichannel Scaling (MCS) from a few picoseconds to several seconds. The FluoTime 300 provides superior sensitivity, spectral, and temporal resolution that allow the acquisition and analysis of
- Fluorescence Spectra
- Fluorescence Lifetime Decay
- Phosphorescence Lifetime Decay
- Fluorescence Anisotropy
- Absolute Quantum Yield
- Emission-Excitation Matrix (EEM)
- Time-Resolved Photoluminescence (TRPL)
- Time-Resolved Emission Spectra (TRES)
from UV to IR range. This setup can be equipped with numerous sample holders ranging from standard cuvette and front face to wafer check. The sample compartment is designed to be spacious to provide more flexibility. For measurements under controlled temperatures, PicoQuant offers various solutions depending on the temperature range.
All components are being controlled by EasyTau 2 software. Unique feature of this spectroscopy software:
- Wizard mode provides step by step assistance
- Customized mode enables full control over the instrument
- Scripting mode allows automation of measurement routines.
The FluoTime 300 is an optimal choice not only for routine lab work but also for cutting-edge research fields. Over 450 research articles have been published utilizing the FluoTime 300 in various fields including solar cells, light emitting diodes (LEDs), photocatalysis, upconversion materials, and quantum dot characterization.
Recent publications by researchers using the FluoTime 300
 Virtual show day: Closer look at the FluoTime series
Virtual show day: Closer look at the FluoTime series
Our first FluoTime Show Day was held on June 17, 2021. During this virtual event, an in-depth introduction on PicoQuant spectroscopy products was provided by our application specialists. Request the recording and learn how our products can help you in your daily research tasks to carry out steady-state and time-resolved luminescence measurements with a micrometer sized positionable observation volume.
 Course on time-resolved fluorescence
Course on time-resolved fluorescence
PicoQuant annually holds the European short course on "Principles and Applications of Time-resolved Fluorescence Spectroscopy". The course is intended for individuals wishing an in-depth introduction to the principles of fluorescence spectroscopy and its applications to the life sciences. For details see the course website.
| Optical configuration |
|
| Mode of operation |
|
| Sensitivity | Signal-to-noise ratio (SNR) > 32 000:1 measured using double monochromators in excitation and emission light paths, PMA Hybrid 06 detector |
| Lifetime range |
Depending on light source and detector choice |
| Excitation sources |
|
| Monochromators |
* Others on request |
| Detectors |
|
| Software |
|
All Information given here is reliable to our best knowledge. However, no responsibility is assumed for possible inaccuracies or omissions. Specifications and external appearances are subject to change without notice.
Specialized sample holders
 Time-resolved and steady-state photoluminescence measurements with the FluoMic
Time-resolved and steady-state photoluminescence measurements with the FluoMic
The FluoMic photoluminescence microscope coupled to the FluoTime 300 provides a fast and easy way to perform time-resolved as well as steady-state photoluminescence measurements on a wide range of solid objects outside of the spectrometer. This set-up extends the power and capabilities of the FluoTime 300 with the ability to gather data from a well defined observation spot that can be freely placed.
Read more on the widefield microscope FluoMic product page >
Read about all options of combining spectroscopy and microscopy >
 Advanced automation for time-resolved spectroscopy
Advanced automation for time-resolved spectroscopy
FluoTime 300 can be coupled to third-party accessories such as the liquid handling automation workstation Biomek NXP from Beckman-Coulter. This combination allows to extend the automation to the loading and removal of samples from the spectrometer, simplifying the work flow for both high-throughput applications and obtaining spectroscopic snap shots at well defined time points.
Check out the demonstration video.
Solar Cells and Photovoltaics
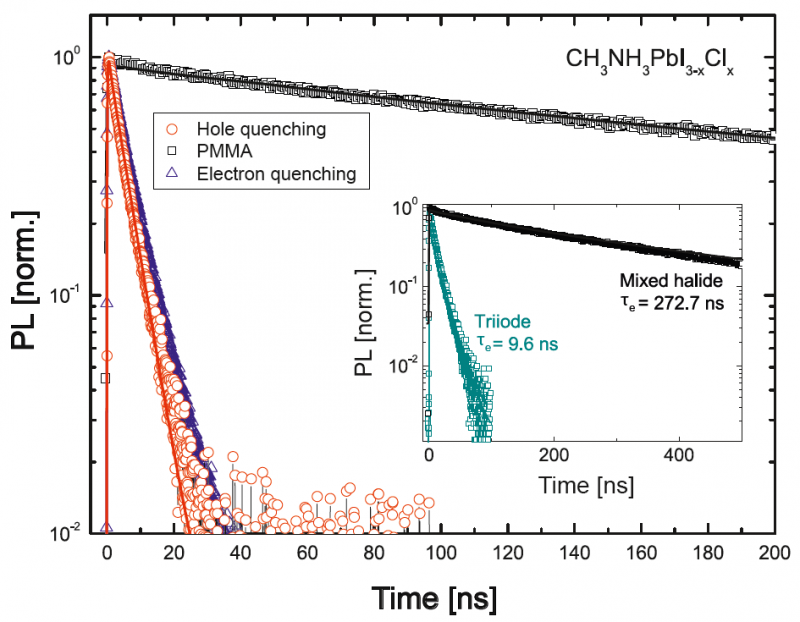 A critical parameter in understanding the photophysics of semiconductor solar cells is the diffusion length of the photo-excited electrons and holes in the material. Time-resolved photoluminescence (TRPL) quenching experiments are a valuable tool for determining diffusion lengths. The example shows data obtained from mixed halide and triiodide organometal perovskite layers in presence of either an electron (blue) or hole (red) quenching layer, or a PMMA coating (black). The decay curves were recorded at 780 nm, corresponding to the peak emission of both materials. The measured decay dynamics can be fitted to a diffusion model, allowing to derive diffusion lengths. Here, the diffusion length of the electrons and holes in the mixed halide perovskite was 1 μm while the triiodide material featured a much shorter length of 100 nm, correlating well with performance of these materials as solar cells.
A critical parameter in understanding the photophysics of semiconductor solar cells is the diffusion length of the photo-excited electrons and holes in the material. Time-resolved photoluminescence (TRPL) quenching experiments are a valuable tool for determining diffusion lengths. The example shows data obtained from mixed halide and triiodide organometal perovskite layers in presence of either an electron (blue) or hole (red) quenching layer, or a PMMA coating (black). The decay curves were recorded at 780 nm, corresponding to the peak emission of both materials. The measured decay dynamics can be fitted to a diffusion model, allowing to derive diffusion lengths. Here, the diffusion length of the electrons and holes in the mixed halide perovskite was 1 μm while the triiodide material featured a much shorter length of 100 nm, correlating well with performance of these materials as solar cells.
[S. D. Stranks et al., Science, 2013]
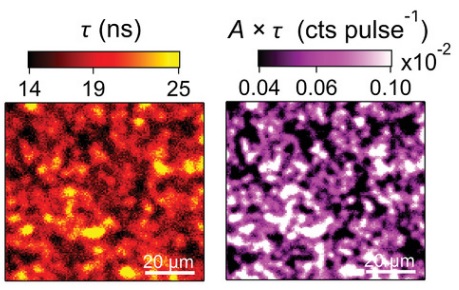 Ochoa et al. used the coupling of confocal microscope MicroTime 100 and spectrometer FluoTime 300 to perform photoluminescence as well as TRPL microscopy on Cu(In,Ga)Se2 solar cells. The charge carrier lifetime is one of the most critical parameters directly affecting VOC. In this study, the effect of inhomogeneities, postdeposition treatments (PDTs) and growth on charge carrier lifetime is investigated.
Ochoa et al. used the coupling of confocal microscope MicroTime 100 and spectrometer FluoTime 300 to perform photoluminescence as well as TRPL microscopy on Cu(In,Ga)Se2 solar cells. The charge carrier lifetime is one of the most critical parameters directly affecting VOC. In this study, the effect of inhomogeneities, postdeposition treatments (PDTs) and growth on charge carrier lifetime is investigated.
[Advanced Energy Materials, 2021]
LEDs, OLEDs
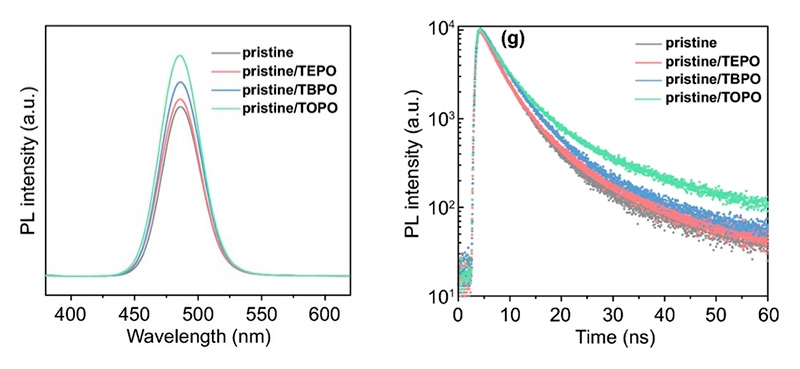 The application of passivating agents to suppress perovskite defects has been extensively investigated. Zhenwei Ren et al. demonstrates tributylphosphine oxide (TBPO) as a decent passivator for the quasi-2D perovskites achieving high-efficient blue PeLED with external efficiency up to 11.5% and operational stability as long as 41.1 min without any shift of electroluminescence. They used FluoTime 300 to acquire photoluminescence (PL) as well as TRPL spectra of perovskite films.
The application of passivating agents to suppress perovskite defects has been extensively investigated. Zhenwei Ren et al. demonstrates tributylphosphine oxide (TBPO) as a decent passivator for the quasi-2D perovskites achieving high-efficient blue PeLED with external efficiency up to 11.5% and operational stability as long as 41.1 min without any shift of electroluminescence. They used FluoTime 300 to acquire photoluminescence (PL) as well as TRPL spectra of perovskite films.
Upconversion Materials
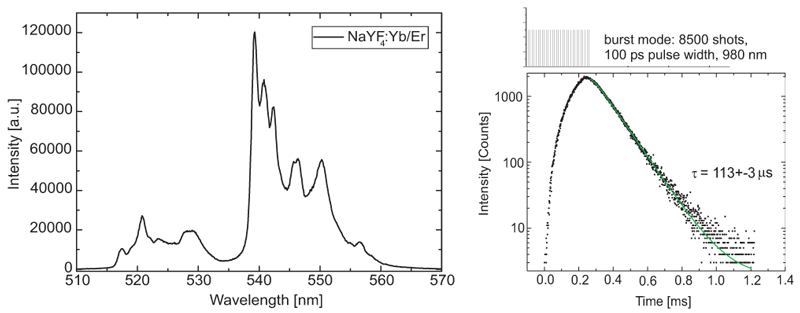
Upconversion materials have diverse applications including optical thermometry, bio-imaging and solar cells. Upconversion nanoparticles (UCNPs) comprising of rare earth elements are among the most studied materials in this field. FluoTime 300 is employed to measure both steady-state and TRPL of NaYF4:Yb/Er solution in cyclohexane.
To acquire the long lifetime of NaYF4:Yb/Er, burst mode is applied that is a unique feature of PicoQuant pulsed light sources enabling fast measurements. The analysis reveals a single fluorescence lifetimes of 113 µs.
Sample courtesy of T. Nyokong, Rhodes University, South Africa
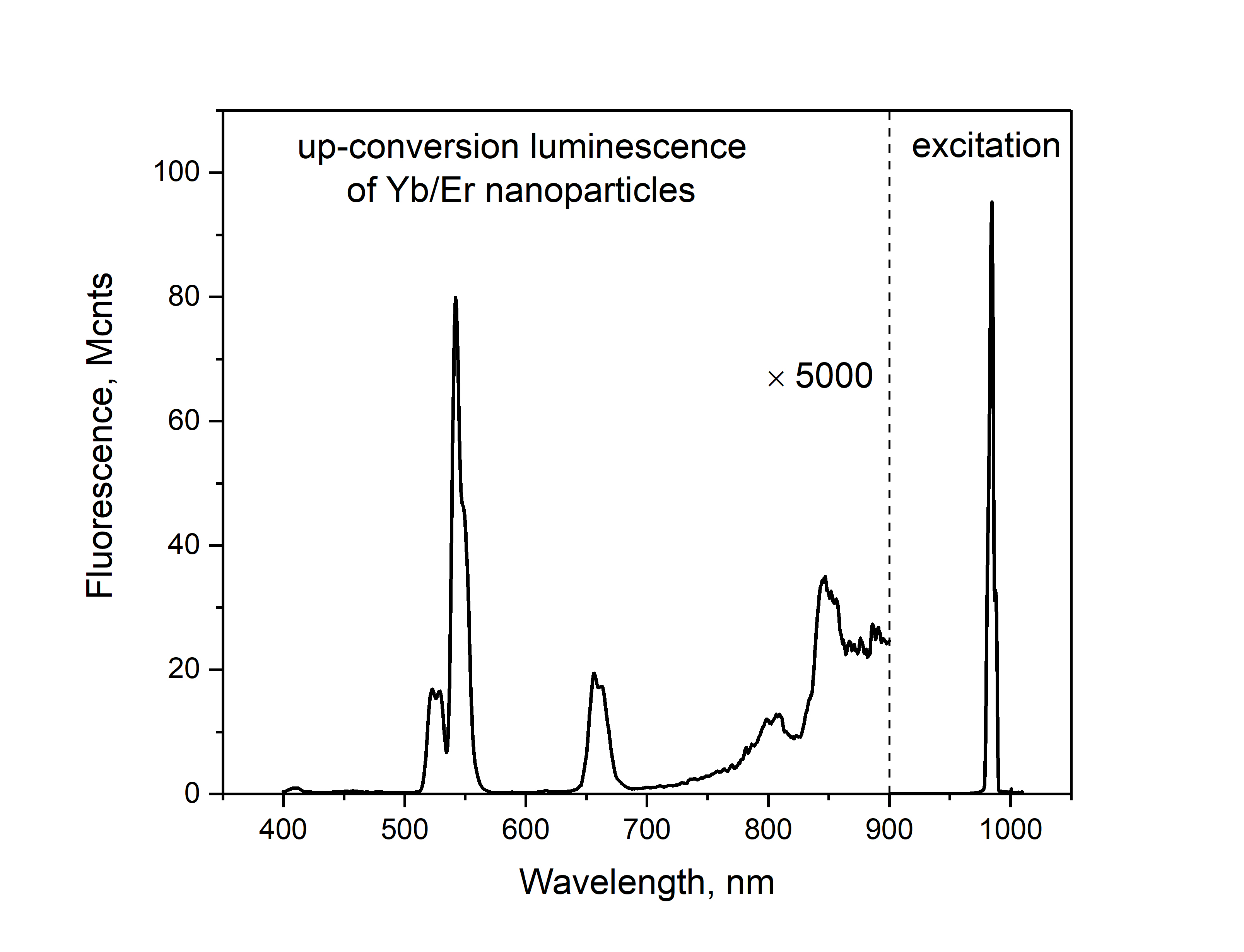
In addition, FluoTime 300 enables the detection of the excitation peak at 980 nm (picosecond laser diode head LDH-D-C-980) that allows the quantum yield measurements. This has been performed on Er/Yb nanoparticles dissolved in cyclohexane.
Sample courtesy of Dr. U. Resch-Genger, BAM
Dyes and Fluorophores
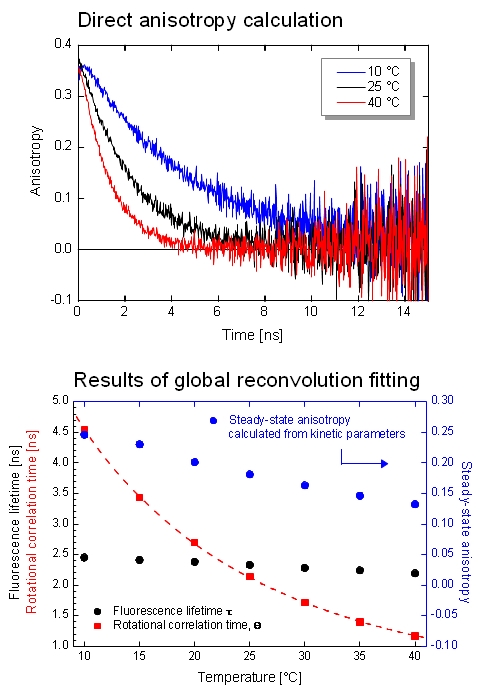 The FluoTime 300 is applied to investigate the dynamic anisotropy of dyes and fluorophores. As an example, the temperature-dependent anisotropy of Coumarin 6 has been studied through automatic measurements of IRF, VV (parallel), VH (perpendicular), and VM (magic angle) polarized decays at various temperature steps via 440 nm excitation. A quick analysis of VV and VH decays clearly shows temperature dependent behavior of the emission anisotropy. The steady-state anisotropy is additionally acquired which were found to be in perfect agreement with the anisotropy values estimated by the Perrin equation.
The FluoTime 300 is applied to investigate the dynamic anisotropy of dyes and fluorophores. As an example, the temperature-dependent anisotropy of Coumarin 6 has been studied through automatic measurements of IRF, VV (parallel), VH (perpendicular), and VM (magic angle) polarized decays at various temperature steps via 440 nm excitation. A quick analysis of VV and VH decays clearly shows temperature dependent behavior of the emission anisotropy. The steady-state anisotropy is additionally acquired which were found to be in perfect agreement with the anisotropy values estimated by the Perrin equation.
Photodynamic Therapy
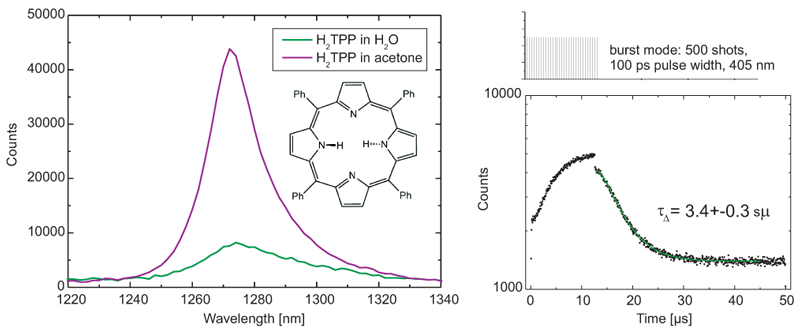
Singlet oxygen refers to an excited state of molecular oxygen. Due to its reactive properties, singlet oxygen is being used to destroy cancer cells in photodynamic therapy. The detection of this chemical requires high sensitivity in near infrared (NIR) range. FluoTime 300 is applied to measure steady-state and time-resolved fluorescence of singlet oxygen emission produced by H2TTPS in acetone and water. The latter is extremely challenging due to the weak emission. To acquire fast lifetime decay, burst mode is used that is a unique feature of PicoQuant pulsed light sources. A tail fit results in lifetime of 3.4± 0.3 µs, which is in excellent agreement with the published literature value.
Images are used, adapted and reproduced under CC BY licence of each corresponding journals.
The following documents are available for download:
- Brochure about the FluoTime Series
- Datasheet FluoTime 300
- Datasheet FluoMic
- Technical note: Time-Correlated Single Photon Counting (TCSPC)
- White Paper: Measuring steady-state and time-resolved photoluminescence from a positionable, micrometer-sized observation volume with the FluoMic
- Application Note: Time-resolved photoluminescence and electroluminescence characterization of a quantum dot LED using the FluoTime 300 spectrometer
- Application Note: Microvolume measurements on FluoTime spectrometer
- Application Note: Time-resolved Fluorescence Spectroscopy and Microscopy in Materials Science
Latest 10 publications referencing FluoTime 300
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this product in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.


 Contact us
Contact us