
Materials Science
Time-Resolved Photoluminescence (TRPL)
Advanced Material Characterization
Time-Resolved Photoluminescence (TRPL) is a powerful technique for investigating the optical and electronic properties of materials by measuring photoluminescence decay times following pulsed excitation. This method enables precise analysis of fluorescence lifetimes, which typically span from picoseconds to milliseconds, depending on the material and its environment.
TRPL reveals key insights into processes such as charge carrier dynamics, defect states, and local emission behavior, making it indispensable in the characterization of nanomaterials and 2D materials such as quantum dots and transition metal dichalcogenides (TMDs).
As a key tool in materials science, TRPL plays a central role in optimizing next-generation technologies – including semiconductor characterization, solar cell research and LED characterization.
Available TRPL Platforms
Various types of set-up can be used for TRPL measurements. We offer a range of modular platforms for time-resolved photoluminescence (TRPL) measurements – covering spectrometer-based, microscope-based, and hybrid configurations. All systems support picosecond to millisecond lifetime resolution and can be tailored to meet specific requirements in materials research.
Click on a category below to explore available TRPL systems and find the setup that best matches your application.
Spectrometers:
FluoTime 300
High-end Photoluminescence Spectrometer
 High-end photoluminescence spectrometer for steady-state and time-resolved measurements (TRPL) from UV to NIR. Offers picosecond to second lifetime resolution, superior sensitivity, and modular upgrades – designed to support advanced materials research focusing on solar cells, quantum dots, 2D materials and more.
High-end photoluminescence spectrometer for steady-state and time-resolved measurements (TRPL) from UV to NIR. Offers picosecond to second lifetime resolution, superior sensitivity, and modular upgrades – designed to support advanced materials research focusing on solar cells, quantum dots, 2D materials and more.
FluoTime 250
Compact Liftetime Fluorometer
Compact and modular fluorescence lifetime spectrometer for steady-state and time-resolved photoluminescence (TRPL) measurements. Offers picosecond to second lifetime resolution, flexible filter-based emission selection, and optional UV-Vis monochromator – ideal for routine and advanced materials research including solar cells, LEDs, and nanomaterials.
Spectrometers
Microscopes:
FluoMic
Compact Upright Widefield Photoluminescence Microscope
Compact upright widefield microscope for time-resolved photoluminescence (TRPL) measurements.
Offers fast and reliable acquisition of steady-state and time-resolved luminescence from UV to NIR, with spatial resolution down to 2 μm – ideal for material screening, quality control, and comparative TRPL studies.
MicroTime 100
Compact Upright Photoluminescence Microscope
Compact and flexible confocal microscope for time-resolved photoluminescence (TRPL) applications. Offers picosecond to microsecond lifetime resolution, broad spectral coverage, and upgrade options such as SNSPD integration, temperature control, and charge carrier diffusion modules – best suited for materials research from solar cells to single emitters.
MicroTime 200
Modular Confocal Photoluminescence Microscope
High-end confocal microscope system for advanced time-resolved photoluminescence (TRPL) studies—down to the single-photon level. Enables true confocal TRPL imaging with picosecond to millisecond lifetime resolution, dual-channel detection, and full modular upgradeability – especially effective for demanding material science research involving quantum dots, 2D materials, and photonic nanostructures.
Microscopes
Micro-Photoluminescence Upgrade

By coupling microscopes and spectrometers, both high-resolution steady-state and time-resolved emission spectra can be acquired from specific regions or points of interest within a sample. Combining expertise from both fields, we offer powerful micro-photoluminescence upgrade options for advanced material analysis. These hybrid configurations enable spatially, spectrally, and temporally resolved TRPL measurements – ideal for probing photophysical behavior. These hybrid configurations enable spatially, spectrally, and temporally resolved TRPL measurements – ideal for probing photophysical behavior. Whether investigating thin-film inhomogeneities, semiconductor interfaces, or localized emission from nanostructures, these solutions deliver maximum flexibility and performance.
An overview of possible configurations can be found on our MicroPL upgrade page.
FlexLambda
Redefining Spectral Selection in Fluorescence Microscopy
 FlexLambda is a compact, software-controlled module for selecting detection wavelengths in TRPL microscopy – without manual filter changes. Fully integrated into PicoQuant’s widefield and confocal systems, it enables fast, reliable acquisition of steady-state and time-resolved photoluminescence across a broad spectral range. By streamlining spectral selection, FlexLambda boosts efficiency and simplifies emission scanning, TRPL measurements, and TRES data acquisition – all without interrupting your experiment.
FlexLambda is a compact, software-controlled module for selecting detection wavelengths in TRPL microscopy – without manual filter changes. Fully integrated into PicoQuant’s widefield and confocal systems, it enables fast, reliable acquisition of steady-state and time-resolved photoluminescence across a broad spectral range. By streamlining spectral selection, FlexLambda boosts efficiency and simplifies emission scanning, TRPL measurements, and TRES data acquisition – all without interrupting your experiment.
Micro-Photoluminescence Upgrade
Applications for TRPL
TRPL of gummy bears, solar cells and LED displays
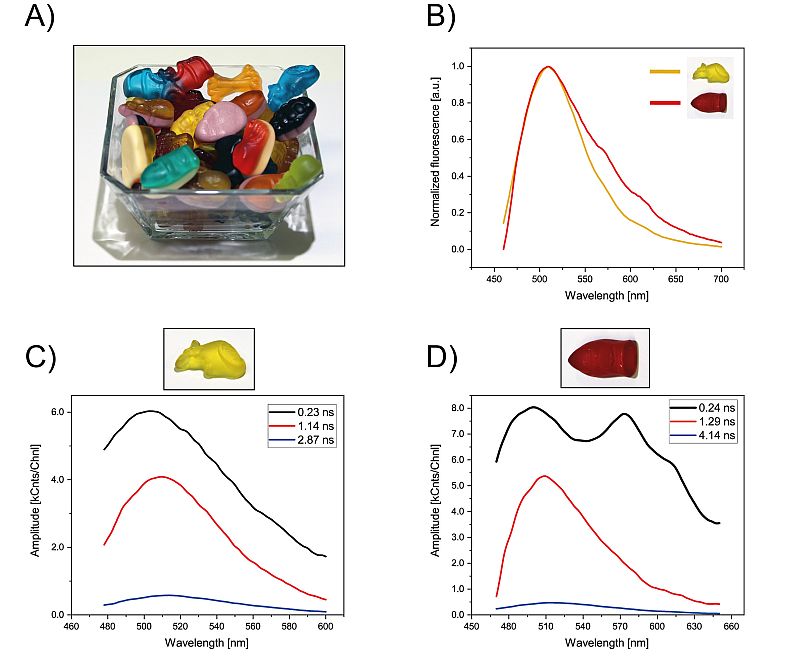 From playful demonstrations to high-tech materials: this example set highlights how TRPL can reveal lifetime differences across vastly different sample types: colorful gummy bears, CIGS solar cells, and individual pixels of an LED smartphone display.
From playful demonstrations to high-tech materials: this example set highlights how TRPL can reveal lifetime differences across vastly different sample types: colorful gummy bears, CIGS solar cells, and individual pixels of an LED smartphone display.
While the gummy bear experiment offers a light-hearted introduction to lifetime spectroscopy, the studies on solar cells and LEDs highlight TRPL’s ability to resolve spatial variations in emission lifetime and material quality.
Together, these examples illustrate how TRPL – especially when combined with spatial resolution using a compact upright widefield microscope FluoMic – can be applied across a wide range of materials.
Set-up:
- High-End photoluminescence spectrometer FluoTime 300 equipped with a compact upright widefield microscope FluoMic
- Excitation: 440 nm with a picosecond pulsed laser head from the LDH Series
TRPL and TREL of a quantum dot LED (QD-LED)
 Improve the energy efficiency and color quality of modern LEDs by gaining deep insights into their luminescence properties. This example highlights how combining steady-state emission spectra with time-resolved photoluminescence (TRPL) and electroluminescence (TREL) analysis provides a complete picture of quantum dot LED (QD-LED) performance.
Improve the energy efficiency and color quality of modern LEDs by gaining deep insights into their luminescence properties. This example highlights how combining steady-state emission spectra with time-resolved photoluminescence (TRPL) and electroluminescence (TREL) analysis provides a complete picture of quantum dot LED (QD-LED) performance.
While spectral measurements reveal overlapping emission from QDs and LED die, time-resolved techniques uncover dynamic processes like carrier lifetimes and recombination mechanisms.
All measurements were performed with the FluoTime 300 spectrometer, demonstrating how such techniques support the development and optimization of next-generation optoelectronic devices.
Set-up:
TRPL for Charge Carrier Analysis in Solar Cells
 Understanding the diffusion length of photo-excited electrons and holes is key to optimizing the performance of solar cell materials –including perovskite-based systems. This example demonstrates how TRPL quenching experiments – combined with selective electron or hole transport layers – enable precise determination of these diffusion lengths.
Understanding the diffusion length of photo-excited electrons and holes is key to optimizing the performance of solar cell materials –including perovskite-based systems. This example demonstrates how TRPL quenching experiments – combined with selective electron or hole transport layers – enable precise determination of these diffusion lengths.
Data recorded from mixed-halide and triiodide perovskite layers show clear differences in decay dynamics, which correlate with device efficiency. This example references a study by Stranks et al. (Science 2013) and is included as part of a broader discussion in the application note below.
Set-up:
Reference: S. D. Stranks et al., Science, 342 (2013), p.341
TRPL Mapping of Layered Semiconductors and Quantum Wells
 How do different layers within a semiconductor wafer contribute to its overall emission behavior?
How do different layers within a semiconductor wafer contribute to its overall emission behavior?
In this example, TRPL combined with spectral and spatial resolution uncovers distinct carrier lifetimes across a GaAsP quantum well structure – revealing variations tied to specific emission bands and physical regions of the sample.
This approach enables detailed mapping of recombination pathways, diffusion effects, and interface quality, making it an essential tool for wafer-level diagnostics and semiconductor development. Since the photoluminescence of semiconductors is a directly related to the charge carrier dynamics, TRPL using TCSPC is highly suited for their analysis and characterization, down to the sub-nanosecond time scale.
Set-up:
- Compact upright photoluminescence microscope MicroTime 100
- High-End photoluminescence spectrometer FluoTime 300
- Excitation: 635 nm with LDH-P-C-635B using a PDL 828
- Detection: PDM 1CTC-SPAD-Detector, detection wavelength > 664 nm
Reference: V. Buschmann et al., 2012 SEMI Advanced Semiconductor Manufacturing Conference, Saratoga Springs, NY, USA, 2012, pp. 187-191.
TRPL Reveals Defect Passivation in High-Efficiency Perovskite Minimodules
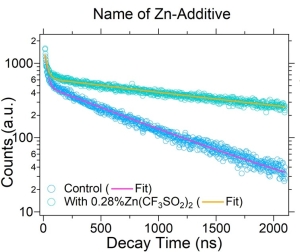 How can tiny molecular additives lead to better large-scale solar devices? This study explores how zinc salts, specifically Zn(OOSCF3)2, reduce iodide-related defects in blade-coated perovskite films. TRPL measurements reveal a ~3x increase in carrier lifetime and enhanced PL quantum yield – direct indicators of successful trap passivation. These insights guided the fabrication of minimodules with certified efficiencies of up to 19.6% over ~80 cm2, setting a new record for this class of devices.
How can tiny molecular additives lead to better large-scale solar devices? This study explores how zinc salts, specifically Zn(OOSCF3)2, reduce iodide-related defects in blade-coated perovskite films. TRPL measurements reveal a ~3x increase in carrier lifetime and enhanced PL quantum yield – direct indicators of successful trap passivation. These insights guided the fabrication of minimodules with certified efficiencies of up to 19.6% over ~80 cm2, setting a new record for this class of devices.
- High-End photoluminescence spectrometer FluoTime 300
- Compact upright photoluminescence microscope MicroTime 100
Reference: M.A. Uddin et al., Nat Commun 15, 1355 (2024).
Latest 10 publications related to TRPL
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this application and our products in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.

 Contact us
Contact us





