Fluorescence Microscopes
SymPhoTime 64
Fluorescence Lifetime Imaging and Correlation Software
- Powerful 64bit TTTR data acquisition and analysis software
- Point, 2D, and 3D data acquisition with online preview of FLIM, FCS, time-traces, or TCSPC histograms
- FLIM, fast FLIM, FLIM-FRET
- FCS, FCCS, FLCS, PIE-FCS, coincidence correlation, total correlation
- FRET, PIE-FRET
- Fluorescence time trace analysis, single molecule burst analysis
- Anisotropy
- TCSPC lifetime fitting with advanced error treatment
- User programming script language "STUPSLANG"
- Description
- Specifications
- Version features
- Videos
- Applications
- Images
- Documents
- Publications
Contact us
The SymPhoTime 64 software package is an integrated solution for data acquisition and analysis using PicoQuant's time-resolved confocal microscope MicroTime 200, MicroTime 100, LSM upgrade kits or TCSPC electronics. Its clearly structured layout and powerful analysis routines allows the user to focus on the results rather than on the data processing. The software is designed for a 64 bit operation system and features a graphical user interface (GUI), which guides the user through all necessary steps for an individual analysis or measurement process. Data dependencies are directly visible in the underlying workspace concept. An integrated scripting language ("STUPSLANG") even puts the user in a position to freely add new analysis procedures or customize existing ones.
Data acquisition with PicoQuant TCSPC modules
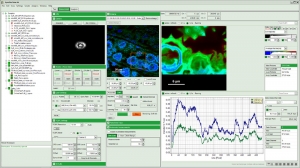 The SymPhoTime 64 is the dedicated data acquisition software for PicoQuant's time-resolved confocal microscopes MicroTime 200 and MicroTime 100 and LSM upgrade kits. It can of course also be used with custom set-ups based on PicoQuant TCSPC electronics. All data acquisition and analysis features of the SymPhoTime 64 are based on the unique time-tagging modes of the MultiHarp 150, TimeHarp 260, PicoHarp 300, PicoHarp 330 or HydraHarp 400. In these modes, photons on each detection channel are tagged with the absolute arrival time since the beginning of the measurement and, in certain cases, also along with the time difference to the last laser pulse. This scheme preserves all photon timing information and allows a large range of data interpretation ranging from simple TCSPC histograms to complex imaging and correlation analysis.
The SymPhoTime 64 is the dedicated data acquisition software for PicoQuant's time-resolved confocal microscopes MicroTime 200 and MicroTime 100 and LSM upgrade kits. It can of course also be used with custom set-ups based on PicoQuant TCSPC electronics. All data acquisition and analysis features of the SymPhoTime 64 are based on the unique time-tagging modes of the MultiHarp 150, TimeHarp 260, PicoHarp 300, PicoHarp 330 or HydraHarp 400. In these modes, photons on each detection channel are tagged with the absolute arrival time since the beginning of the measurement and, in certain cases, also along with the time difference to the last laser pulse. This scheme preserves all photon timing information and allows a large range of data interpretation ranging from simple TCSPC histograms to complex imaging and correlation analysis.
Adapted interfaces for imaging analysis
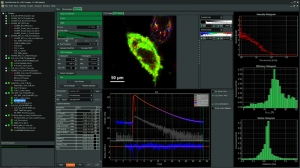
 With the SymPhoTime 64, analysis of (time-resolved) imaging measurements will be easier than ever before. The software provides special adapted interfaces for many standard analysis procedures ranging from Fluorescence Lifetime Imaging (FLIM) to Fluorescence Resonance Energy Transfer (FRET) and Anisotropy. Each interface only makes those procedures available that are directly required for the individual analysis. This ensures a steep learning curve as well as quick and correct analysis results.
With the SymPhoTime 64, analysis of (time-resolved) imaging measurements will be easier than ever before. The software provides special adapted interfaces for many standard analysis procedures ranging from Fluorescence Lifetime Imaging (FLIM) to Fluorescence Resonance Energy Transfer (FRET) and Anisotropy. Each interface only makes those procedures available that are directly required for the individual analysis. This ensures a steep learning curve as well as quick and correct analysis results.
Ultrafast software correlator
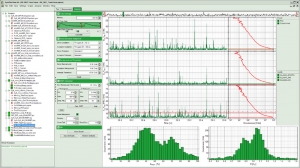
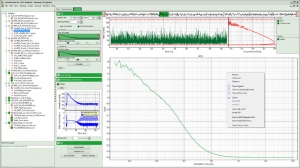 The SymPhoTime 64 also sets a new standard for analysis of fluorescence correlation spectroscopy measurements. The software provides a wide range of specially adapted correlation analysis procedures, which range from classical auto-correlation (FCS) and cross-correlation (FCCS) to lifetime based correlation analysis (FLCS) and total correlation. Even coincidence-correlation analysis is possible based on the unique time-tagging modes of the PicoQuant TCSPC modules. By exploiting the full power of a multi-core computer system, the SymPhoTime 64 is one of the fastest software correlators on the market.
The SymPhoTime 64 also sets a new standard for analysis of fluorescence correlation spectroscopy measurements. The software provides a wide range of specially adapted correlation analysis procedures, which range from classical auto-correlation (FCS) and cross-correlation (FCCS) to lifetime based correlation analysis (FLCS) and total correlation. Even coincidence-correlation analysis is possible based on the unique time-tagging modes of the PicoQuant TCSPC modules. By exploiting the full power of a multi-core computer system, the SymPhoTime 64 is one of the fastest software correlators on the market.
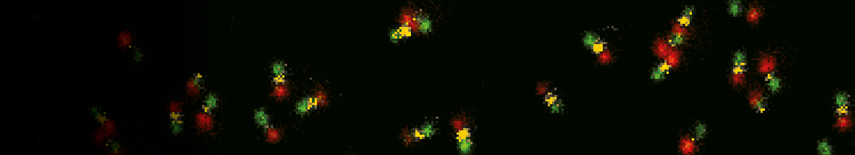
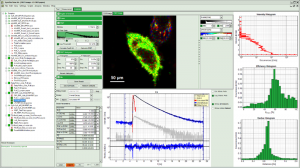
Intensity time trace analysis
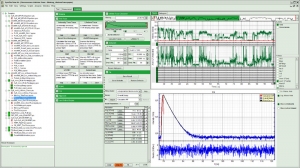 The analysis of fluorescence intensity time traces is another core feature of the SymPhoTime 64. Fluorescence intensity time traces display the measured fluorescence dynamics and can be analysed in a variety of ways. Prominent examples are classical single molecule methods such as on/off histograms, burst size histograms or fluorescence lifetime traces.
The analysis of fluorescence intensity time traces is another core feature of the SymPhoTime 64. Fluorescence intensity time traces display the measured fluorescence dynamics and can be analysed in a variety of ways. Prominent examples are classical single molecule methods such as on/off histograms, burst size histograms or fluorescence lifetime traces.
STED super-resolution
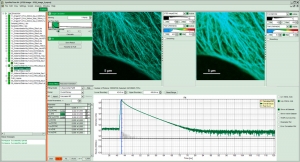 The SymPhoTime 64 supports the super-resolution STED add-on for the MicroTime 200. Different data acquisition modes including interleaved trains of excitation and STED laser pulses are integrated in the GUI. STED images as well as time-gating for gSTED are displayed online during data acquisition. Multiple image processing approaches such as gSTED and Pattern Matching are implemented for even further improvement of the resolution. STED-FCS is also available.
The SymPhoTime 64 supports the super-resolution STED add-on for the MicroTime 200. Different data acquisition modes including interleaved trains of excitation and STED laser pulses are integrated in the GUI. STED images as well as time-gating for gSTED are displayed online during data acquisition. Multiple image processing approaches such as gSTED and Pattern Matching are implemented for even further improvement of the resolution. STED-FCS is also available.
Different packages for different requirements
The software is available in five different packages in order to meet the different needs of the individual users:
- SPT64 1A "SymPhoTime 64": for analysis of 1-D measurements (stationary beam / sample)
- SPT64 1 "SymPhoTime 64": for 1-D data acquisition and analysis (stationary beam / sample)
- SPT64 2 "SymPhoTime 64": for 2-D and 3-D data acquisition and analysis
- SPT64 1+2 "SymPhoTime 64": complete version
- SPT64 1+2+3 "SymPhoTime 64": complete version for STED
Current software version: 2.9
The latest version adds support for the PicoHarp 330 with 4 detection channels and the FlexLambda Wavelength Selection Unit for Confocal Microscopes
| Data Acquisition | |||
|---|---|---|---|
| Supported TCSPC modules | HydraHarp 400, PicoHarp 300, PicoHarp 330, TimeHarp 260, MultiHarp 150, MultiHarp 160, TimeHarp 200 (Data import ONLY!) | ||
| Supported Configurations | MicroTime 200 with 100 x 100 (x 100) µm or 10x7.5 cm piezo scanner or FLIMbee galvo scanner MicroTime 100 Laser Scanning Microscopes (LSM)from Nikon, Olympus or Zeiss Stand-alone TCSPC modules Remote control via TCP/IP interface (software handshake with ZEN and NIS Elements) |
||
| Routing | 1 to 8 detectors | ||
| Measurement modes | Single point, multi-point, 2D imaging (XY, XZ, YZ), 3D imaging (XYZ), time lapse (XYT), oscilloscope mode for alignment purposes | ||
| Measurement previews | FLIM, FCS, FLCS and FCCS, Time Trace, TCSPC histogram Parallel calculation and display of up to 4 different previews |
||
| Automated Measurements | Z–Stacks, Time Stacks, Image Stitching, Multi-Point Measurements | ||
| Hardware control | PDL 828 "Sepia II" laser driver E-710, E-725, E-727 and wide range scanner controller (Physik Instrumente) Shutter of MicroTime 200 Wide-field fluorescence camera IDS uEye USB3 in MicroTime 200 |
||
| Data Analysis | |||
| General Features | Time gating Binning TCSPC Binning TCSPC Fitting (Multi–Exponential Decay (1 to 5 Exponentials), Least–Squares Fitting, MLE Fitting, IRF Reconvolution, Tailfit, Bootstrap error analysis) Global analysis of TCSPC curve fitting GUI Themes |
||
| Imaging | FLIM, FLIM-FRET, Intensity FRET, Anisotropy Imaging, (Time-Gated) Fluorescence Intensity Imaging Pattern Matching, Fast Pattern Matching Adjustable color scale Region-of-Interest (ROI) Bin export for phasor analysis (via third party software Globals developed by the Laboratory for Fluorescence Dynamics) |
||
| Correlation | FCS, FCCS, FLCS, PIE–FCS STED-FCS, STED-FLCS FCS Fitting (Models: Diffusion Constants, Triplet state, Conformational, Protonation, Gaussian PSF, User-defined models via scripting, Bootstrap error analysis) Global analysis FCS calibration Antibunching/Coincidence correlation, Total correlation |
||
| FRET | PIE (Pulsed Interleaved Excitation) Bleedthrough Correction FLIM-FRET |
||
| STED | STED, gatedSTED, STED-FLIM, Pulsed Interleaved STED and Confocal, Resolution estimation | ||
| Fluorescence Intensity Traces | Blinking (On/Off Histogramming), Count Rate Histogram (PCH), Burst Size Histogram, Intensity–Gated TCSPC, Fluorescence Lifetime Traces, Lifetime Histogram, BIFL (Burst Integrated Analysis | ||
| Steady–State Anisotropy | Objective correction factors included | ||
| Export data formats | BMP, ASCII, TIFF, BIN | ||
| User Scripting (STUPSLANG) | User–Defined Analysis Procedures, Fitting Functions, Multi–Parameter Filtering Control of external hardware via suited interface |
||
| Required PC CPU |
with SSE2 and EMT64 or AMD64 extension; recommended: quad–core or better | ||
| CPU clock | 2.2 GHz (or better) quad-core CPU | ||
| RAM | minimum 4 GB (suggested 16/32 GB) | ||
| Operating system | Windows 10 x64 | ||
| Disk space | >= 100 MB (except data storage) | ||
| Display(s) | Full HD Display | ||
| Protection Module (HASP) | USB | ||
All Information given here is reliable to our best knowledge. However, no responsibility is assumed for possible inaccuracies or omissions. Specifications and external appearances are subject to change without notice.
The main features of the four versions are shown in the following table:
| SPT64 1A | SPT64 1 | SPT64 2 | SPT64 1+2 | SPT64 1+2+3 | |
| Data Acquisition Features | |||||
|---|---|---|---|---|---|
| Direct data acquisition in TTTR mode using MultiHarp 150/160, TimeHarp 260, PicoHarp 300, PicoHarp 330 or HydraHarp 400 |
 |
 |
 |
 |
|
| Control of Physik Instrumente scanner |  |
 |
 |
||
| Support for Laser Scanning Microscopes (LSM) and generic scanners via external trigger signals |
 |
 |
|||
| STED imaging, STED-FLIM, STED-FCS |  |
||||
| Router support (separation of up to eight detector signals) |  |
 |
 |
 |
 |
| Multi point measurements |  |
 |
|||
| Data Analysis Features | |||||
| Time gating for all methods |  |
 |
 |
 |
 |
| TCSPC-lifetime histogramming and fitting to exponential decay functions up to 5th order incl. tail fitting, reconvolution analysis, maximum likelihood estimation and bootstrap error analysis |
 |
 |
 |
 |
 |
| Fluorescence Lifetime Imaging (FLIM) incl. online-visualisation of FLIM data during the measurement, arbitrary regions of interest for analysis, maximum likelihood estimation and bitmap export |
 |
 |
 |
||
| Pattern Matching, Fast Pattern Matching |  |
 |
 |
||
| Fluorescence Correlation Spectroscopy (FCS) incl. Pulsed-Interleaved Excitation (PIE) treatment, (auto- or cross correlation) & FCS fitting with bootstrap error analysis |
 |
 |
 |
 |
|
| Fluorescence Lifetime Correlation Spectroscopy (FLCS) |  |
 |
 |
 |
|
| FCS calculation and time trace display during the measurement ("online time traces", "online-FCS") |
 |
 |
 |
||
| Total correlation |  |
 |
 |
 |
|
| Förster Resonance Energy Transfer (FRET) incl. Pulsed-Interleaved Excitation (PIE) treatment, corrections for direct excitation and bleedthough |
 |
 |
 |
 |
|
| STED imaging, STED FLIM, gatedSTED, Pulsed Interleaved STED and Confocal, Resolution estimation |  |
||||
| Multi-channel scaling analysis – Lifetime and intensity time traces |
 |
 |
 |
 |
|
| Burst integrated fluorescence lifetime (BIFL) analysis |  |
 |
 |
 |
|
| On/Off time histogramming and analysis |  |
 |
 |
 |
|
| Burst size histogramming |  |
 |
 |
 |
|
| Calculation of Photon Counting Histogramm usable for PCH |
 |
 |
 |
 |
|
| Generic ASCII export filter |  |
 |
 |
 |
|
| Scripting ("STUPSLANG") |  |
 |
 |
 |
 |
SymPhoTime 64 - Software feature demonstration
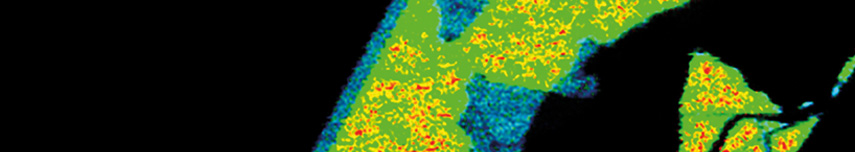
3D FLIM z-stack of Daisy pollen
Measure an Instrument Response Function (IRF) with a LSM Upgrade Kit
Performing a FCS measurement with a LSM upgrade kit
Performing a FLIM-FRET Measurement with a LSM Upgrade Kit
Measuring a Fluorescence Lifetime Image (FLIM) with a LSM Upgrade Kit
Recording a Fluorescence Lifetime Image (FLIM) Stack with a LSM Upgrade Kit on a Nikon A1
The Fluorescence Lifetime Imaging and Correlation Software SymPhoTime 64 can be used in all applications that are based on time-resolved confocal data acquisition, such as:
- Time-Resolved Fluorescence
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Foerster Resonance Energy Transfer (FRET)
- Stimulated Emission Depletion Microscopy (STED)
- Dual Focus Fluorescence Correlation Spectroscopy (2fFCS)
- Pulsed Interleaved Excitation (PIE)
- Single Molecule Spectroscopy / Detection
- Pattern Matching Analysis
- Time-Resolved Photoluminescence (TRPL)
- TRPL Imaging
- Lanthanide Upconversion
- Antibunching
The following documents are available for download:
- Brochure about the SymPhoTime 64 software
- Pattern matching analysis using SymPhoTime 64
- Practical Manual for Fluorescence Microscopy Techniques
Presentations (as PDF files)
The following step-by-step tutorials for the SymPhoTime 64 are available at http://www.tcspc.com
- Script registration
- Focal width determination
- Calibrate the confocal volume using the FCS calibration script
- TCSPC fitting script
- Lifetime fitting using the FLIM script
- Lifetime separation using the FLCS script
- Using the intensity time trace script
- Caluclate and fit FCS traces with the FCS script
- Calculate FCCS traces with the grouped FCS script
- Using the FLCS script for spectral crosstalk removal via FLCS
- ROI-fitting using the FLIM script
- Visualizing dynamics using the multi frame FLIM script
- Pattern matching
- FLIM-FRET calculation for multi-exponential donors
- FLIM-FRET calculation for single-exponential donors
- Calculate ratiometric FRET images with the FRET image script
- Single-pair FRET distributions using the FRET script
- Single-pair FRET distributions using the PIE-FRET script
- Using the anisotropy image script
- Using the antibunching script
Latest 10 publications referencing MicroTime 200, LSM Upgrade Kit, MicroTime 100, SymPhoTime
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this product in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.