Single Molecule Workshop
29th International Workshop on “Single Molecule Spectroscopy and Super-resolution Microscopy”
October 8 - 10, 2024, Berlin, Germany

Meet the single molecule community in Berlin!
The 29th edition of the Single Molecule Workshop will take place from Ocotober 8 – 10, 2024. We are looking forward to welcoming you in Berlin. Join us for an exciting and stimulating conference by either giving a talk, presenting a poster or even without any presentation. As always, we will be awarding a “Best Student Talk” prize worth 750 Euro.

Aim and purpose
The focus of PicoQuant’s long-standing workshop lies on ultrasensitive optical detection down to the single molecule level as well as beyond the classical diffraction limit. The event provides an interdisciplinary platform for exchanging ideas and recent results between researchers and professionals working in the fields of physics, chemistry, biology, life and materials science.
During the workshop, talks and posters are presented that cover a wide range of applications and methods revolving around the challenging field of Single Molecule Spectroscopy.
Covered topics include:
- Fluorescence Lifetime Imaging (FLIM)
- Single molecule Förster Resonance Energy Transfer (smFRET)
- Polarization and Anisotropy based techniques
- Quantitative imaging methods
- New fluorescence sensors and labeling schemes
- Fluorescence Correlation Spectroscopy (FCS) and Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Stimulated Emission Depletion (STED) microscopy
- Single Molecule Localization Microscopy (e.g., PALM, STORM, dSTORM, GSDIM, PAINT)
- Open source data analysis solutions
- Big Data and Machine Learning approaches to superresolution and single molecule techniques
Both widefield and confocal fluorescence microscopy techniques are covered as well as in vivo and in vitro measurements with single molecule sensitivity.
Important dates
- Deadline for abstract submission: July 14, 2023
- Deadline for early bird registration: July 14, 2023
- Deadline for fee waiver application: July 14, 2023
- Final deadline for workshop registration: August 25, 2023
- Notification on acceptance of abstracts: August 2023
- Program available: August 2023
Demos of Luminosa, our new confocal microscope
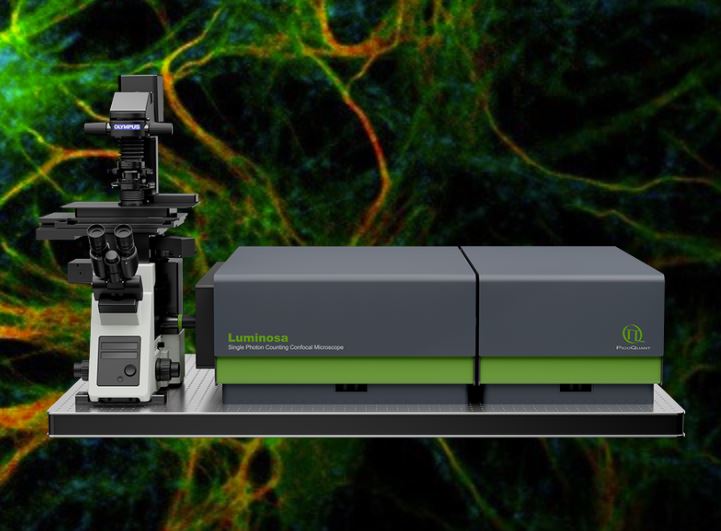 During the workshop we offer demos of our new single photon counting confocal microscope Luminosa.
During the workshop we offer demos of our new single photon counting confocal microscope Luminosa.
You can reserve a slot when you are registered for the workshop.
Student Award
 As nurturing young scientists is important to PicoQuant, we host a competition for the “Best Student Talk” with an award worth 750 Euro.
As nurturing young scientists is important to PicoQuant, we host a competition for the “Best Student Talk” with an award worth 750 Euro.
Submission is closed.
Conference on Single Molecule Spectroscopy at BiOS 2024
 Within the Biomedical Optics Symposium BiOS, PicoQuant is co-organizing the special conference "Single Molecule Spectroscopy and Superresolution Imaging XVII" (BO503). Expect exciting presentations with the latest findings and developments.
Within the Biomedical Optics Symposium BiOS, PicoQuant is co-organizing the special conference "Single Molecule Spectroscopy and Superresolution Imaging XVII" (BO503). Expect exciting presentations with the latest findings and developments.
As a special motivation for young researchers, PicoQuant is presenting the "Young Investigator Award" as part of this conference. Young scientists (age 30 or below and not yet full faculty members) are encouraged to participate in this best paper competition, which offers a $750 cash award.
Next workshop (preliminary date)
- October 8-10, 2024
Contact
Workshop coordinator: Claudia Bergemann
Tel: +49-30-1208820-87
Fax: +49-30-1208820-90
Email: workshop@picoquant.com
Invited speakers and their tentative titles
 Suzanne A. Blum
Suzanne A. Blum
University of California, Irvine USA
 Anindya Datta
Anindya Datta
IIT Bombay, India
 Lars Hubatsch
Lars Hubatsch
Max Planck Institute of Molecular Cell Biology and Genetics, Germany
 Sergio Padilla-Parra
Sergio Padilla-Parra
King’s College London, United Kingdom
 Emmanuel Margeat
Emmanuel Margeat
Centre de Biochimie Structurale, Montpellier, France
 Susana Rocha
Susana Rocha
Katholieke Universiteit Leuven, Belgium
 Quan Wang
Quan Wang
National Institutes of Health, USA
 Stefanie Weidtkamp-Peters
Stefanie Weidtkamp-Peters
Heinrich-Heine University Duesseldorf, Germany Jennifer Lippincott-Schwartz
Jennifer Lippincott-Schwartz
Howard Hughes Medical Institute, USA
Registration
Registration will open in spring 2024.
Archive
The workshop on "Single Molecule Spectroscopy and Ultra Sensitive Analysis in the Life Sciences" is an annual event since 1995. To get an impression of our Single Molecule Workshops have a look at the video below.
For a summary of each year's event, please select the year from the list below.
Thank you for registering for the Single Molecule Workshop!
An email with the supplied information has been sent to the provided address.
×
